A global total of 206.030 people with multidrug- or rifampicin-resistant TB (MDR/RR-TB) were detected and notified in 2019, a 10% increase from 186.883 in 2018. About half of the global burden of MDR-TB is in 3 countries – India, China and the Russian Federation.
(World Health Organization)
Tuberculosis (TB) is one of the most important infectious diseases and thus a topic of global importance. A significant reduction of the TB incidence or complete elimination of TB is not unrealistic. Therefore, the article provides important information on the topic of TB and, at the same, important background information on the fascinating biology of the causal agent of TB, Mycobacterium tuberculosis (Mtb). In order to be able to fight TB effectively, innovative diagnostic approaches are needed – especially in the field of Point-of-Care (PoC) applications. For this reason the article focuses on the high requirement profile of comprehensive TB diagnostics and what impact the universal Lateral Flow Development Platform Milenia HybriDetect can have.
Mycobacterium tuberculosis – the causative agent of TB
Mycobacterium tuberculosis (Mtb) is a rod-shaped, gram-positive, non-motile bacterium that belongs to the actinobacteria, one of the most diverse divisions of the bacterial domain. Many people know Mtb as the etiological agent of one of the most important infectious diseases – tuberculosis (TB). TB is an airborne disease that belongs to the Top 10 causes of death worldwide and it is present in every country and age group. According to WHO data, around 1.4 Mio. people died from TB in 2019. Mycobacterial drug resistance in particular is one of the most important issues in the context of TB diagnosis, medication and prevention.
Mtb has extremely interesting characteristics that underscore its pathogenic potential and explain why TB is a serious global threat. In contrast to many other pathogenic bacteria, Mtb is able to remain in the host organism for a long time without causing significant damage. In a first host reaction, Mtb cells can be enclosed in special structures, the so-called granuloma. But Mtb proves to be very adaptable and can change its metabolism and thus survive in the host for a long time. In this latent phase, when the host is not contagious, Mtb proves to be a “master of manipulation”. For example, proteins that modify or imitate the host’s signal pathways are secreted in a very targeted manner.
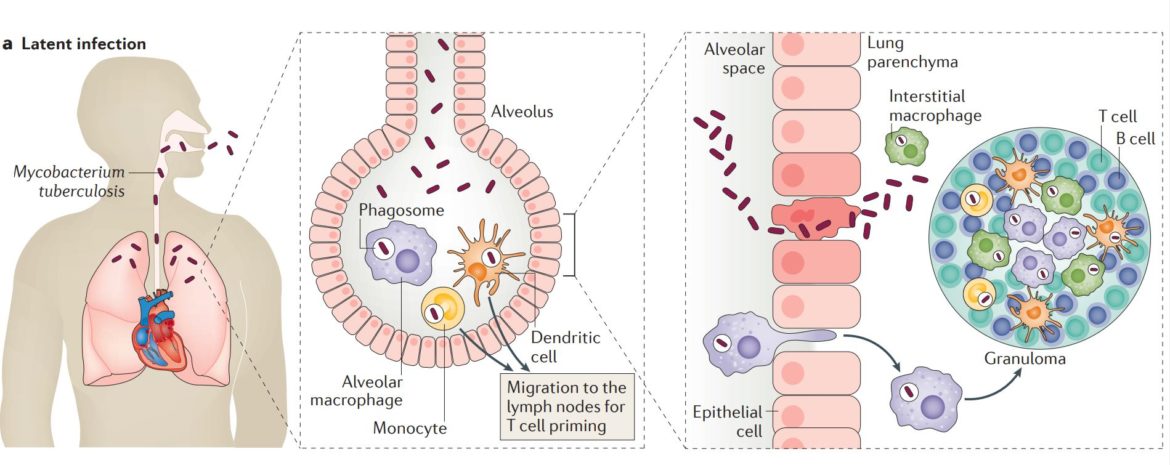
But if the host’s immune system is weakened, e.g. due to medication or virus infection, the mycobacteria quickly adapt and begin to divide. At a certain point, Mtb can no longer be retained in the granuloma structures and the bacteria are released. From this point on mycobacteria cause TB-active disease. The host develops characteristic symptoms and thus is able to infect others.
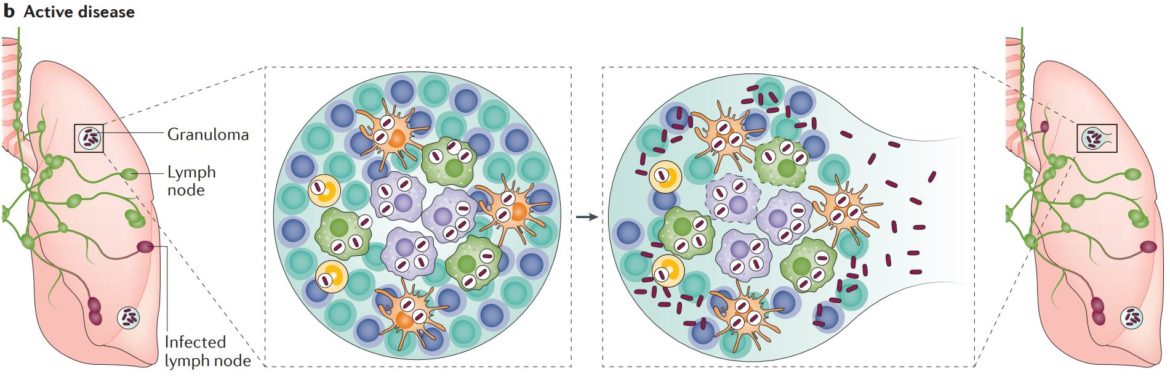
Beside common symptoms (cough with sputum and blood at times, chest pains, weakness, weight loss, fever and night sweats) the role of Mtb as major risk factor for other lung diseases (lung cancer, COPD), autoimmune diseases or metabolic syndromes (obesity, diabetes, atherosclerosis) is intensively discussed in science.
Learn more – Madhukar Pai et al., 2016 – Excellent Review on Tuberculosis
Drug Resistant Mycobacterium tuberculosis
Mycobacteria are without a doubt fascinating. However, some characteristics of these pathogens make the airborne disease TB a very serious health security threat. Basically, TB is a disease that can be cured efficiently with medication. But this is only the case for drug-susceptible Mtb strains. Unfortunately, it is not uncommon for Mtb to develop resistance(s) to overcome potent drugs. This is very often due to the misuse or mishandling of anti-TB drugs, but also due to the biology of the mycobacteria themselves. A basic distinction is made between monoresistant TB, multi-resistant TB and extensively drug resistant TB. The following definitions are cited from the CDC-homepage.
- Monodrug-resistant TB is caused by an Mtb strain that is resistant to one of the first line anti-TB drugs.
- Multidrug-resistant TB (MDR TB) is caused by an Mtb strain that is resistant to at least isoniazid and rifampin, the two most potent TB drugs. These drugs are used to treat all persons with TB disease.
- Extensively drug-resistant TB (XDR TB) is a rare type of MDR TB that is resistant to isoniazid and rifampin, plus any fluoroquinolone and at least one of three injectable second-line drugs (i.e., amikacin, kanamycin, or capreomycin). Because XDR TB is resistant to the most potent TB drugs, patients are left with treatment options that are much less effective.
Intrinsic & Acquired Resistance
In the case of mycobacterial resistance, a fundamental distinction can be made between intrinsic resistance and acquired resistance. The intrinsic resistance can be traced back to species-specific properties that do not require additional mutations. This includes for example the lipid rich composition of the mycobacterial cell wall, which is often described as waxy. Furthermore, transport systems (efflux pumps) can efficiently remove active substances from the cell and thus lead to drug tolerance or resistance. However, enzymatic drug modification or inactivation are also important mechanisms of mycobacterial intrinsic resistance. On the other hand, there is the so-called acquired resistance. In the presence of an anti-TB drug, bacterial cells are subject to selective pressure. If a point mutation occurs that reduces the effectiveness of the anti-TB drug, a selective advantage arises. In extreme cases, the accumulation of such mutations can lead to multidrug- or extensively drug resistant Mtb strains.
More Information about Drug Resistance in Mycobacteria:
- General Information CDC: https://www.cdc.gov/tb/topic/drtb/default.htm
- General Information WHO: https://www.who.int/news-room/fact-sheets/detail/tuberculosis
- Iacobino et al., 2020 https://www.mdpi.com/2076-3417/10/6/2153
- Dookie et al., 2018 https://academic.oup.com/jac/article/73/5/1138/4817621
- Gygli et al., 2017 https://academic.oup.com/femsre/article/41/3/354/3089982
The Need for Innovative, Rapid & Field-Applicable TB Diagnostics
Although TB can be found in all regions of the world, an overwhelming majority of active TB cases can be traced back to structurally weak regions such as Africa or Southeast Asia. In addition to the development of alternative anti-TB drugs and vaccines, innovations in TB diagnostics are one of the major challenges of the near future in order to actually overcome TB. In particular, the focus must be on test formats that allow simple, rapid and robust analysis. The challenge is to achieve sensitivities and specificities that come close to current laboratory methods. The combination of innovative molecular biology & super simple and robust visualization of the results can be the basis of a new generation of diagnostic tools that could have a significant impact in the field of TB-diagnostics.
“Aside from improving the quality of public health systems in resource-limited settings, novel, cost-effective point-of-care diagnostic tools, drugs and an effective vaccine are urgently needed”
(S.M. Gygli et al., 2017; Swiss Tropical and Public Health Institute; FEMS Microbiology Reviews, fux011, 41, 2017, 354–373)
“Through research and innovation, WHO works to accelerate development of rapid diagnostics and treatments for drug-resistant TB”
(World Health Organization)
“Research work must continue towards developing new molecular and advanced techniques for rapid and accurate diagnosis of TB, with better performance characteristics, that can be easily implemented for routine TB diagnosis in low-resource countries.”
Milenia HybriDetect – A Universal Tool for Field-Applicable, Molecular TB Diagnostics
The universal lateral flow test strips of the Milenia HybriDetect platform are ideal for the visualization of DNA-or RNA-amplification products, but also for the rapid assessment of highly sensitive CRISPR-Cas based detection methods. The demands on modern and comprehensive TB diagnostics are high. It is about safe methods for species identification or the detection of drug resistances. But it is also about techniques for determining host-specific risk factors and associated virus diagnostics (e.g. HIV). In the following list, representative publications are given that deal with TB-associated issues or in which relevant techniques are described.
- Rapid & sensitive Identification of Mycobacterium tuberculosis (complex)
- Point-of-Care Detection of Drug Resistances (e.g. Single-Nucleotide-Polymorphisms)
- Evaluation of Host-specific, Risk Factors (e.g. genetic predispositions, viral infections)
The universal Lateral Flow Development Platform Milenia HybriDetect enables equipment free analysis of molecular biological products, even in a multiplex setup. (Isothermal) amplification techniques or innovative CRISPR-Cas-based diagnostics are used frequently. In this „type of application“, the strip is cited again and again in scientific publications, but it is also used as a kit component in commercial POC test systems. But our universal test strips can do a lot more …
Learn more about general detection principles using Milenia HybriDetect.
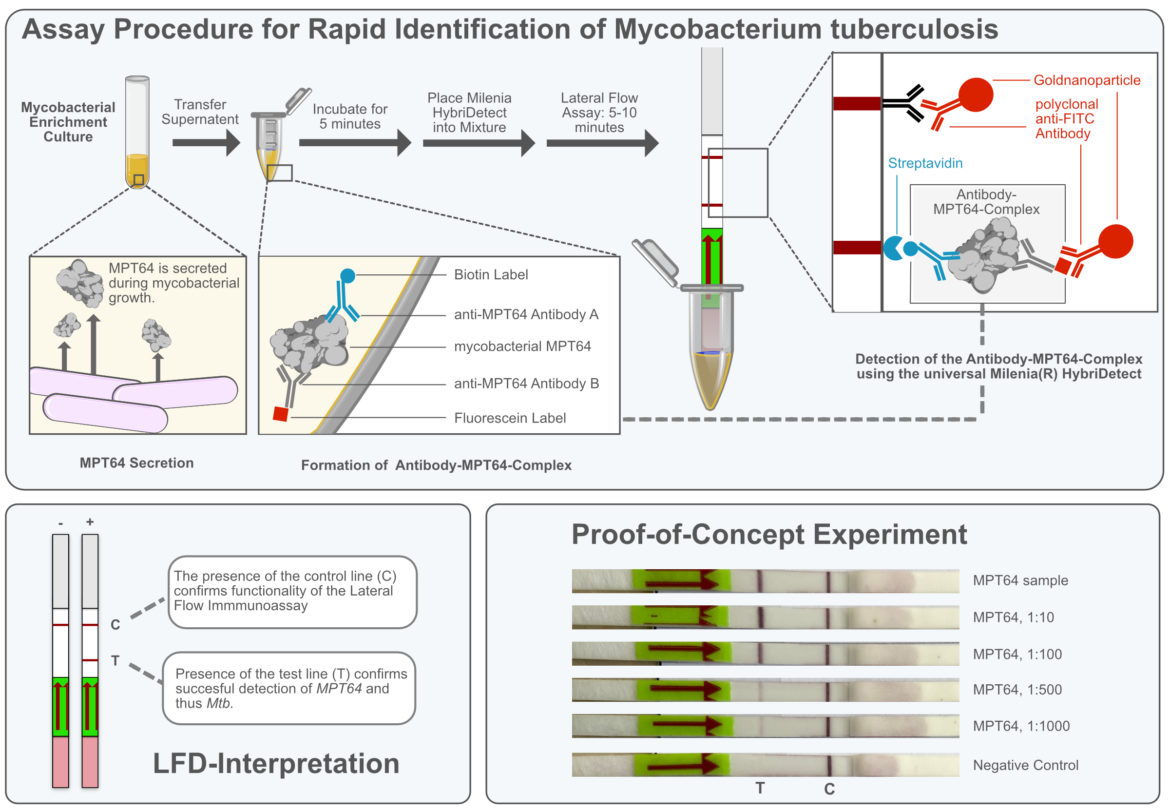
Milenia HybriDetect – A Platform for the Detection of Proteins & Nucleic Acids
Ultimately, the functional principle is based on the recognition of an analyte that is labeled with biotin and FITC / FAM / Fluorescein. In internal experiments, we want to test how well the Milenia HybriDetect is suitable as a development platform for antibody-based rapid tests. Our aim was to provide an extremely simple and robust test system for the Point-of-Care identification of Mtb from an enrichment culture without DNA-/RNA-amplification. A pair of monoclonal anti-MPT64 antibodies were biotinylated and fluorescein-labeled, respectively. MPT64 is a Mtb-characteristic protein that is secreted in large quantities by the bacterium into the culture medium. Thus, culture supernatant can be used as sample matrix for the analysis, in order to confirm the presence of Mtb. After co-incubation of the sample with the anti-MPT64-antibody cocktail, the Milenia HybriDetect test strip is directly placed in the reaction tube. Mycobacterial growth is confirmed, if a clearly recognizable test line appears within 5 to 10 minutes. Once the antibodies were available, the test system was successfully established within a single lab day. We will try to provide a detailed summary of these experiments soon. The following figure shows the basic functionality.
Outlook
TB is still an important topic that threatens the whole world. A significant reduction of the TB incidence or complete elimination of TB is not unrealistic. But it requires investing in science in order to find new vaccines and alternative drugs. The third important pillar beside drugs and vaccines is the development of future-oriented diagnostics. TB in particular illustrates how complex the demands of comprehensive future orientated Point-of-Care compatible diagnostics are – from simple and rapid identification of characteristic biomarkers such as the MPT64 to very precise and sensitive analyzes for the detection of resistances or even genetic predisposition of the host.
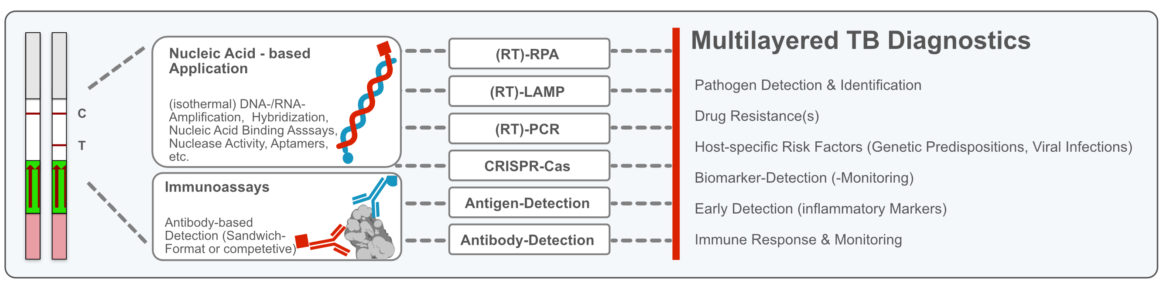
The use of universal lateral flow devices such as the Milenia HybriDetect can make an important contribution to multi-layered point-of-need diagnostics in the future.
- Pai, M., Behr, M. A., Dowdy, D., Dheda, K., Divangahi, M., Boehme, C. C., Ginsberg, A., Swaminathan, S., Spigelman, M., Getahun, H., Menzies, D., & Raviglione, M.: Tuberculosis. Nature reviews. Disease primers, 2, 16076. https://doi.org/10.1038/nrdp.2016.76 (2016)
- Iacobino, A.; Fattorini, L.; Giannoni, F.: Drug-Resistant Tuberculosis 2020: Where We Stand. Appl. Sci. 2020, 10, 2153. https://doi.org/10.3390/app10062153 (2020)
- Navisha Dookie, Santhuri Rambaran, Nesri Padayatchi, Sharana Mahomed, Kogieleum Naidoo: Evolution of drug resistance in Mycobacterium tuberculosis: a review on the molecular determinants of resistance and implications for personalized care, Journal of Antimicrobial Chemotherapy, Volume 73, Issue 5, May 2018, Pages 1138–1151, https://doi.org/10.1093/jac/dkx506 (2018)
- Sebastian M. Gygli, Sonia Borrell, Andrej Trauner, Sebastien Gagneux: Antimicrobial resistance in Mycobacterium tuberculosis: mechanistic and evolutionary perspectives, FEMS Microbiology Reviews, Volume 41, Issue 3, May 2017, Pages 354–373, https://doi.org/10.1093/femsre/fux011 (2017)
- Eddabra, R., Ait Benhassou, H.: Rapid molecular assays for detection of tuberculosis. Pneumonia 10, 4 (2018). https://doi.org/10.1186/s41479-018-0049-2 (2018)
- Joon, D., Nimesh, M., Gupta, S., Kumar, C., Varma-Basil, M., & Saluja, D.: Development and evaluation of rapid and specific sdaA LAMP-LFD assay with Xpert MTB/RIF assay for diagnosis of tuberculosis. Journal of microbiological methods, 159, 161–166. https://doi.org/10.1016/j.mimet.2019.03.002 (2019)
- Jaroenram, W., Kampeera, J., Arunrut, N., Sirithammajak, S., Jaitrong, S., Boonnak, K., Khumwan, P., Prammananan, T., Chaiprasert, A., & Kiatpathomchai, W. : Ultrasensitive detection of Mycobacterium tuberculosis by a rapid and specific probe-triggered one-step, simultaneous DNA hybridization and isothermal amplification combined with a lateral flow dipstick. Scientific reports, 10(1), 16976. https://doi.org/10.1038/s41598-020-73981-6 (2020)
- Ma, Q., Liu, H., Ye, F., Xiang, G., Shan, W., & Xing, W.: Rapid and visual detection of Mycobacterium tuberculosis complex using recombinase polymerase amplification combined with lateral flow strips. Molecular and cellular probes, 36, 43–49. https://doi.org/10.1016/j.mcp.2017.08.004 (2017)
- Myhrvold, C., Freije, C. A., Gootenberg, J. S., Abudayyeh, O. O., Metsky, H. C., Durbin, A. F., Kellner, M. J., Tan, A. L., Paul, L. M., Parham, L. A., Garcia, K. F., Barnes, K. G., Chak, B., Mondini, A., Nogueira, M. L., Isern, S., Michael, S. F., Lorenzana, I., Yozwiak, N. L., MacInnis, B. L., … Sabeti, P. C. : Field-deployable viral diagnostics using CRISPR-Cas13. Science (New York, N.Y.), 360(6387), 444–448. https://doi.org/10.1126/science.aas8836 (2018)
- Osborn, M. J., Bhardwaj, A., Bingea, S. P., Knipping, F., Feser, C. J., Lees, C. J., Collins, D. P., Steer, C. J., Blazar, B. R., & Tolar, J. : CRISPR/Cas9-Based Lateral Flow and Fluorescence Diagnostics. Bioengineering (Basel, Switzerland), 8(2), 23. https://doi.org/10.3390/bioengineering8020023 (2021)
- Crannell, Z. A., Rohrman, B., & Richards-Kortum, R. : Equipment-free incubation of recombinase polymerase amplification reactions using body heat. PloS one, 9(11), e112146. https://doi.org/10.1371/journal.pone.0112146 (2014)
- Rohrman, B. A., & Richards-Kortum, R. R. : A paper and plastic device for performing recombinase polymerase amplification of HIV DNA. Lab on a chip, 12(17), 3082–3088. https://doi.org/10.1039/c2lc40423k (2012)
- : Centers for Disease Control and Prevention (CDC), General Information on Tuberculosis (TB) Website – CDC-TB ()
- : World Health Organization (WHO), Key Facts about Tuberculosis (TB), Website-WHO-TB ()



