“Due to the isothermal character, the robustness against inhibitors and the amplification speed, the RPA has developed to the most relevant DNA amplification method in the field of Point-of-Care applications under resource-limited settings.“
Recombinase Polymerase Amplification & Lateral Flow
A Short Introduction
Recombinase Polymerase Amplification (RPA) is a very sensitive, isothermal DNA amplification method that is extremely robust against common inhibitors and runs at comparatively low temperatures. The RPA can be directly combined with reverse transcription, which allows the sensitive detection of defined RNA sequences.
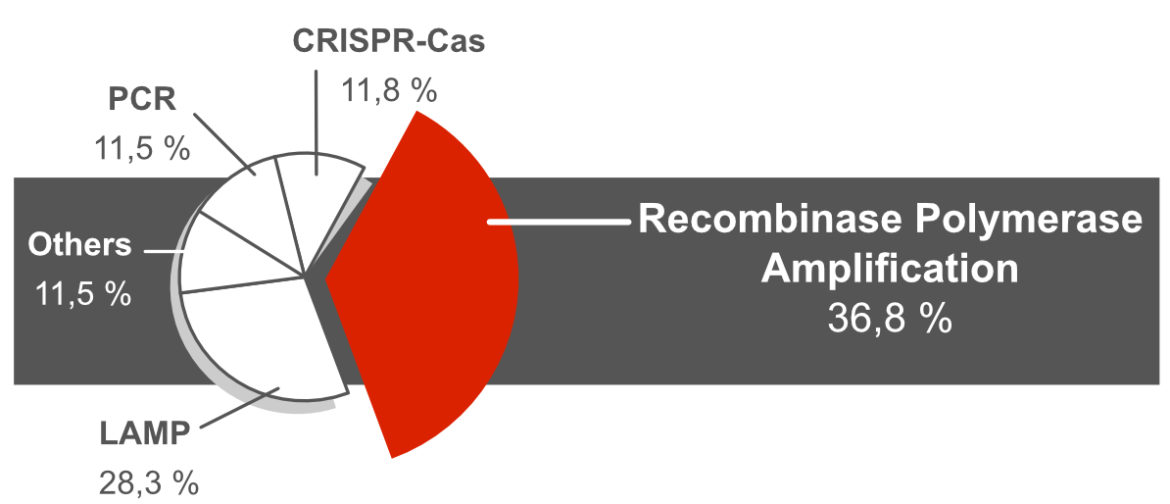
History of the Recombinase Polymerase Amplification
The RPA was initially described in 2006 by Niall Armes and Olaf Piepenburg. The authors of the publication with the title: „DNA Detection Using Recombination Proteins” were already aware of the importance of their innovative DNA amplification method for point-of-care applications. Even then, an RPA method was presented that could be combined with lateral flow analysis using the Milenia HybriDetect. 15 years after publication of this landmark paper, RPA has developed to one of the most frequently used DNA-amplification techniques in combination with universal lateral flow immunoassays.
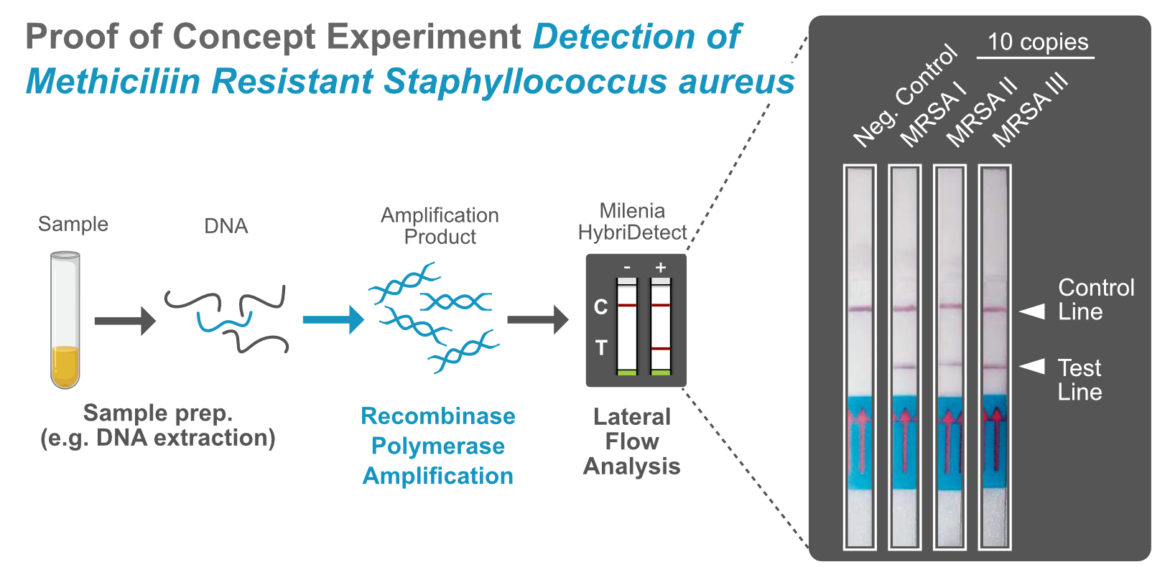
General Mechanism of the Recombinase Polymerase Amplification
The RPA works at temperatures around 37 to 42 °C. In order to achieve exponential amplification at lower temperatures, components of DNA replication or recombination are used to mimic natural processes. Viral proteins, that are involved in DNA-recombination, -repair, and –replication in vivo, are added to the RPA- reaction to allow primer invasion. A suitable DNA-polymerase with strong strand displacement activity ensures elongation and thus efficient amplification of the RPA product.
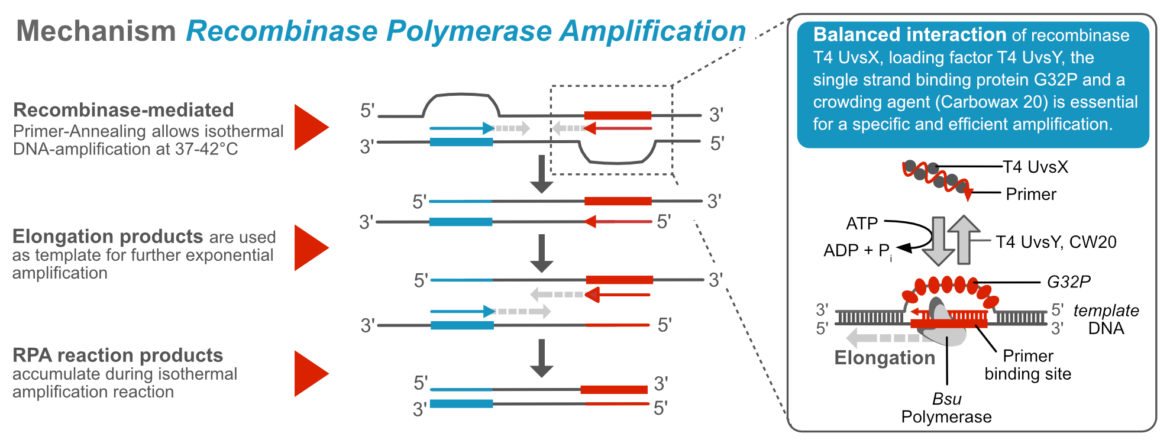
Modification of a RPA for Lateral Flow based Readout
The most important supplier of RPA reagents is TwistDx, which is part of Abbott since 2018. In order to avoid the detection of non specific RPA-products via Lateral Flow, it is highly recommended to add an additional specificity-generating step to the RPA, the Endonuclease IV. TwistDX has so far offered the TwistAmp® nfo kit for this purpose. Since 2022, however, this kit is no longer available so you must recreate it by yourself by just adding endonuclease IV to the TwistAmp® Basic – Kit. How to implement Endonuclease IV is shown by Zirngibl et al. in their publication “A Rapid Molecular Assay for Detecting the Mediterranean Fanworm Sabella spallanzanii Trialed by Non-Scientist Users” in detail. Endonuclease IV can be purchased at several suppliers.
Nfo – Endonuclease IV
The use of an additional enzyme was already described in the first RPA publication in 2006. Armes and Piepenburg described the addtion of the Endonuclease IV (nfo) to the RPA reaction, which solved the problem of non specific amplification during RPA. The nfo-mediated, site-specific cleavage of an elongation blocked probe was neccessary to initiate the efficient amplification of dual labelel amplicons, which were detectable with the universal Lateral Flow Device, Milenia HybriDetect 2T. This addition was essential for a sufficient specificity of the RPA-Lateral Flow rapid test. Nevertheless, this methodological detection strategy complicates the general RPA assay desgin.
Finding the perfect primer-/probe-combination
In order to achieve a satisfying RPA sensitivity, the search for the perfect primer-/ probe-combination can be quite complex. The rules for primer design are comparable to those for PCR, except that the primers should be made significantly longer (30-36 bases) and the amplicons should be kept small. The use of an additional nfo probe (approx. 45 bases) further complicates the assay design. However, this should not be a reason to opt out of the RPA. Ultimately, it is only a matter of investing more time and energy in a meaningful primer-probe-screening system. As a reward, very sensitive, specific amplification reactions can succeed, which run with an impressive robustness and speed even under limited-resource settings.
RPA & Multiplexing
Generally RPA is an isothermal DNA-amplification technique that allows extremely rapid and robust analysis. But RPA is not known to be the perfect technique for multiplexing. Nevertheless, it is possible to develop multiplex RPA assays that are perfectly compatible with the multiplex Lateral Flow Device – Milenia HybriDetect 2T. Multiplex RPA assays have been successfully developed, published and thus provide multiple information under low resource settings. As described recently by Mancuso et al., Anal. Chem. 93, 2021 even (semi-) quantitative analysis is possible due to a special duplex assay design. Moreover, stronger multiplexing can be achieved in combination with additional post amplification techniques, such as nested amplification, simple probe hybridization or CRISPR-Cas-dependent amplicon recognition assays.
Limitations of the RPA
In principle, the RPA is suitable for multiplex applications, but assay design and the general RPA performance noticeably limit the multiplexing ability. In addition to the problem of potential carryover contaminations, the non-specific amplification of unwanted products is one of the most important RPA-characteristic limitations. A Methodical adaptation such as nfo-dependent RPA or final hybridization with an amplicon-specific probe is often unavoidable.
Benefits of the RPA
Recombinase polymerase amplification is one the most important isothermal amplification techniques for a new generation of molecular point-of-care diagnostics. RPA can be incredibly fast. Results in less than 10 minutes are possible with sensitivities that are comparable to established laboratory methods. The isothermal character at temperatures around 37°C, the compatibility with the lateral flow technology and the robustness against inhibitors make the RPA one of the most important tools for sensitive and accurate rapid testing in a true Point-of-Need setting.
| Method combined with LFA | Sensitivity | Specificity | Speed | reverse Transcription (one pot) | Robustness | Method. Variety | Simple Assay Design | Multiplexing Ability | Prevention Carryover Cont. |
|---|---|---|---|---|---|---|---|---|---|
| PCR | |||||||||
| LAMP | |||||||||
| RPA | |||||||||
| CRISPR (Label-Sep.) | |||||||||
| CRISPR (Label-Inc.) |