Another important point is the turnaround time. Isothermal amplification techniques can be extremely fast compared to standard PCR. Results can be generated in 10 – 30 minutes of amplification with an impressive sensitivity.

Two isothermal amplification techniques are currently the most frequently used methods in the field of Lateral Flow-based POC-Analysis. These are the Recombinase Polymerase Amplification (RPA) and the Loop mediated isothermal Amplification (LAMP). The following table shows a comparison of the relevant features of these methods to the most popular DNA-amplification technique, PCR.
| Characteristics | PCR | RPA | LAMP |
|---|---|---|---|
| Isothermal (Temperature) | no | yes (37-42°C) | yes (60-72°C) |
| Low Resource (Equipment) | (+) | +++ | ++ |
| Detection Speed | (++) | +++ | +++ |
| Colirometric Readout | + | + | +++ |
| Lateral Flow based Readout | +++ | ++ | +++ |
| Assay Design | +++ | ++ | + |
| Multiplexing | +++ | + | + |
The table emphasizes that each amplification method has its own characteristic performance profile with certain strengths and weaknesses. The following chapters will focus on one of the most used isothermal amplification methods, the LAMP.
LAMP – Molecular Insights
The name “Loop mediated isothermal Amplification” is a very apt description of the molecular amplification mechanism. The LAMP is more complex than the other amplification methods, such as PCR or RPA. A total of four to six primers are necessary to initiate exponential amplification. Here is an attempt to explain the mechanism relatively simply: In the initial phase of amplification, a dumbbell-shaped reaction intermediate is formed due to the elongation of large modular primers and continuous strand displacement of primary amplicons. The terminal loops of this intermediate structure are the starting point for the exponential amplification during LAMP. The following figure (1) and the video-link are explaining the amplification mechanism in detail.
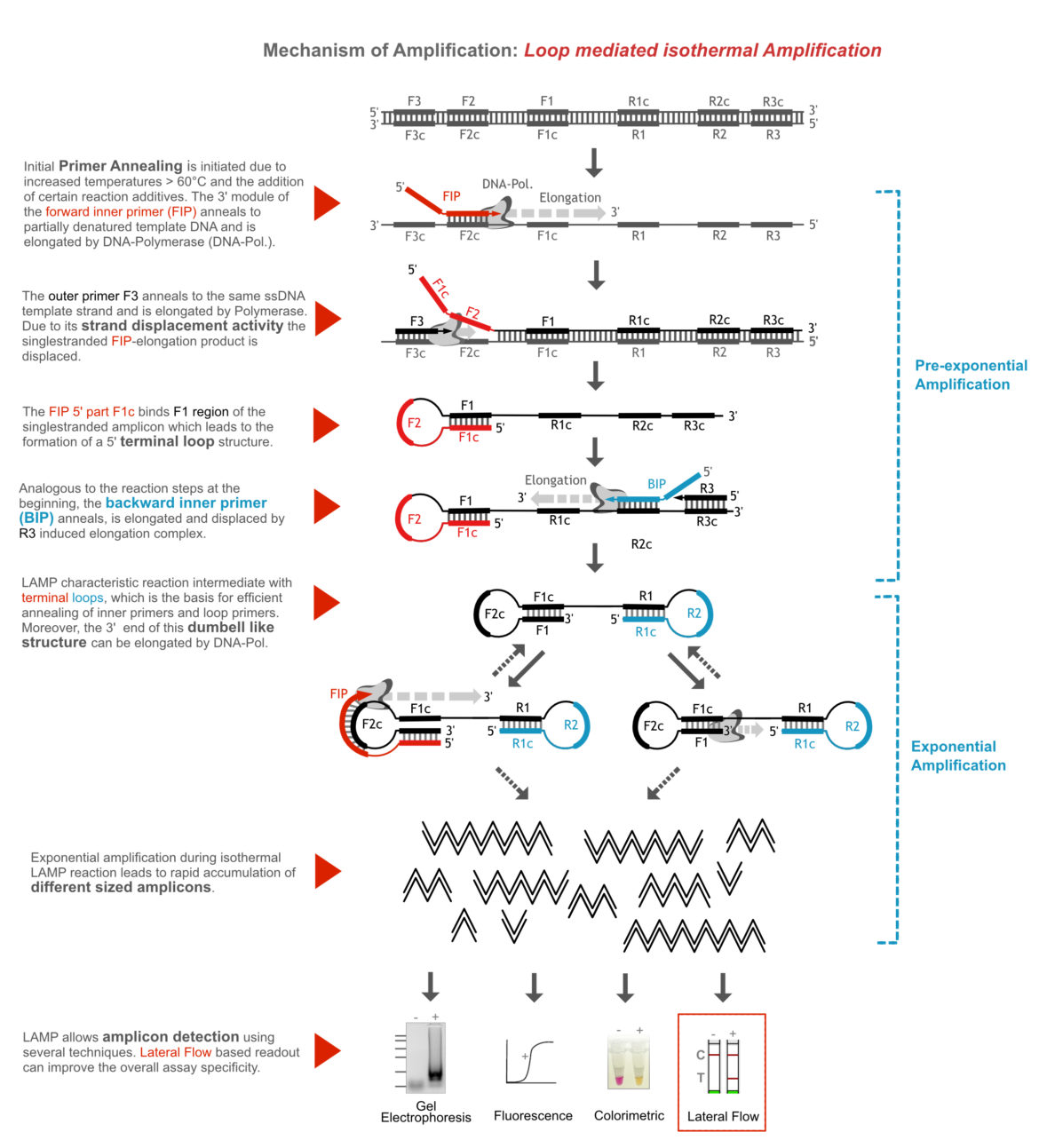
The “working horse” for isothermal Amplification
The characteristic enzyme, which catalyzes the LAMP reaction, is the Bst polymerase. This DNA polymerase originates from Bacillus stearothermophilus and exhibits a strong strand displacement activity and lacks 5 ‘ > 3’ exonuclease activity. To our experience the in silico modified, aptamer blocked Bst 2.0 warmstart polymerase is the perfect tool for easy and fast assay development. Nonspecific amplification is inhibited under 45°C, which allows reaction setup and handling at room temperature. This is the equivalent to the hot start DNA-polymerases used for PCR. Interestingly Bst polymerase exhibits reverse transcriptase activity. The fusion-polymerase Bst 3.0 is optimized for LAMP and is even more suitable for one step RT-LAMP due to the enhanced RT-activity. Taken together the Bst Polymerase is an impressive “working horse” for isothermal amplification from DNA and RNA.
In addition, there are other enzymes that are also suitable for loop mediated isothermal amplification even at higher temperatures. When looking for the perfect LAMP polymerase, it can be worth to take a closer look.
LAMP Readout Strategies
An amplification method or a diagnostic test are often only as good as the associated readout platform. There are a lot of different ways to visualize a LAMP reaction product. Comparable to other DNA-amplification techniques, LAMP products can be detected via agarose electrophoresis or fluorescence detection in a Real-Time cycler. However, these platforms tend to be laborious and require special equipment.
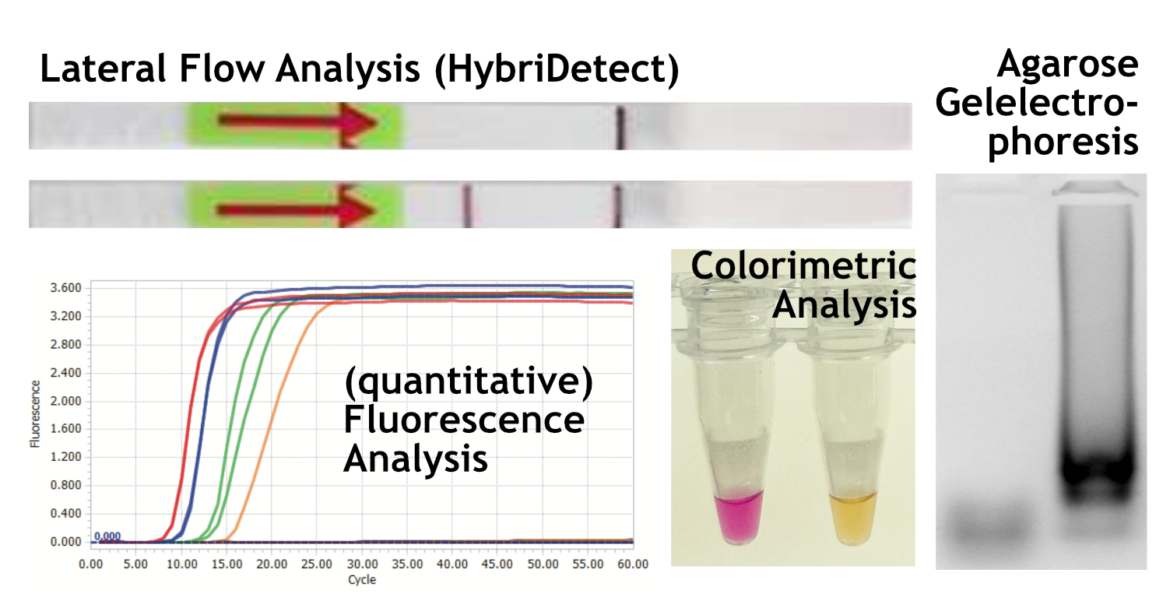
The possibility of evaluating the amplificate with the naked eye is particularly interesting for POC-compatible applications.
A characteristic of this isothermal amplification method is the massive accumulation of reaction products, which qualifies it for simple colorimetric naked eye detection (2). Disadvantages associated with this charming and simple approach are restrictions on the direct sample usage, more complex sample preparation and no chance for a multiplexing. Moreover, nonspecific amplification can result in false positive interpretation of a test. Therefore, the visualization of LAMP-products by Lateral Flow Analysis (LFA) has evolved to a versatile and easy-to-handle alternative. The LAMP-LFA has its strengths in sensitivity, simplicity, multiplex compatibility and, depending on the labeling strategy, an impressive specificity. The Milenia HybriDetect platform is a frequently cited, universal and easy-to-handle Lateral Flow dipstick for individual LAMP-LFA development.
However, each method also has certain weaknesses. In the case of the LAMP-LFA, there are additional working steps compared to direct colorimetric detection. In addition, the amplificate must somehow get onto the LFD, which enables a potential carryover contamination problem. The following table gives an overview of the advantages and disadvantages of each “readout strategy”.
| Advantages/Disadvantages | LAMP-AGE | qRT-LAMP | Colirometric LAMP | LAMP-LFA |
|---|---|---|---|---|
| additional work | +++ | + | - | ++ |
| additional time | +++ (20-30 min) | - | - | + (5-10 min) |
| equipment necessary | ++ | +++ | -/+ | - |
| simplicity interpretation | ++ | ++ | +++ | +++ |
| complexicity sample prep. | + | + | +/++/+++ | + |
| risk contamination (carryover) | ++ | - | - | ++ |
| POC-compatibility | (+) | (+) | +++ | +++ |
In silico Primer Design
In contrast to PCR, the LAMP requires a relatively complex primer design. Four primers are necessary to initiate the LAMP reaction. This includes two loop-forming, modular designed inner primers (FIP and BIP) and two outer primers (F3 and B3), which are necessary for strand displacement of the characteristic FIP- and BIP-related elongation product. Additional loop primers are able to improve the amplification efficiency. Due to the complexity, it is not recommended to design LAMP-Primers by hand. Rather, it makes sense to use available online tools to find an appropriate primer set and use this as a basis for further optimization. Eiken’s PrimerExplorer (V5) and the NEB LAMP – Primer Design Tool are very useful when it comes to LAMP primer design. Lucigen’s webinar video is recommended to learn more about LAMP primer design and assay optimization.
Developing a LAMP-LFA: Labeling Strategies of Primers and CO
One of the major challenges in LAMP-LFA-design is the labeling strategy of the primers, probes or even dNTPs. It is important to understand the impact of the positioning of the required labels, because it has a significant impact on the overall assay LAMP-LFA performance. The following figure gives an overview of a typical LAMP-Primer arrangement.
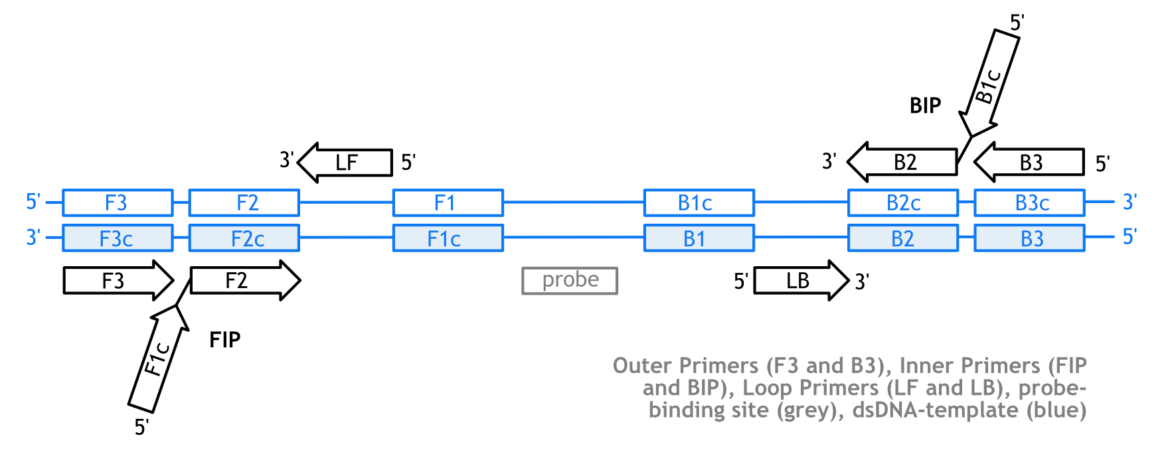
For example, the 5’ labeling of LAMP-associated primers can be extremely effective. This strategy can end up in very sensitive LAMP assays. On the other hand, if nonspecific LAMP-products occur, they eventually become detectable on the LFD. Using a labeled probe can significantly enhance the overall specificity, but depending on the probe design, an additional work step makes the workflow more complicated. Another important factor is the probability of cross primer dimers. Some oligonucleotides tend to interact with each other at lower temperatures, which can result in amplification-independent signals on the LFD. For this reason, the FIP- and BIP-labeling can be critical, especially for multiplex applications. It is recommended to pretest the labeled primer-pair in silico (e.g. Thermo’s Multiple Primer Analyzer) before ordering the oligonucleotides. The following table gives an overview of existing labeling strategies.
| Labeled Component | Label Location | post LAMP Hybridization | Specificity | Propability: Cross Primer Dimers | Multiplexing | Reference Number |
|---|---|---|---|---|---|---|
| FIP and BIP | FIP: 5' Biotin BIP: 5' FITC/FAM | no | ++ | +++ | yes | 3-5 |
| FIP and LF/BIP and BF | FIP/BIP: 5' Biotin LF/BF: 5' FITC/FAM | no | ++ | ++ | yes | 6,7 |
| LF and BF | LF: 5' Biotin BF: 5' FITC/FAM | no | ++ | + | yes | 8 |
| dNTPs and LF or BF | dNTPs: Biotin-11-dUTP LF/BF: 5' FITC/FAM | no | ++ | - | yes | 9 |
| dNTPs only | dNTP-1: Biotin-11-dUTP dNTP-2: FITC-aha-dUTP | no | + | - | no | 10 |
| FIP or BIP and probe | FIP/BIP: 5' Biotin probe: 5' FITC/FAM | yes | +++ | ++ | yes | 11-14 |
| LB and probe (LAMP) | LB: 5' Biotin probe: 5' FITC/FAM, 3'inversed dT | no | +++ | + | yes | 15 |
| FIP and probe (in LAMP) | FIP: 5' Biotin 5' FITC/FAM, 3' spacer C3 | no | +++ | ++ | yes | 16 |
The High-Dose-Hook-Effect: a hidden, but common Pitfall
As mentioned before, a massive amount of reaction product is created in an efficient LAMP-assay. This characteristic can be already noticed in the composition of a LAMP-reaction. Compared to other amplification techniques a huge amount of primers is introduced into the reaction. Especially the inner primers (FIP and BIP) and the loop primers (LF and LB) are used in high concentrations. Depending on the preferred labeling strategy it is quite likely that too many labels are introduced into the LFA. This would lead to a significant loss of sensitivity due to the high dose hook effect. The hook effect is a typical immunoassay related phenomenon. It occurs, if too many LFA-relevant labels (Biotin / FITC / FAM / DIG) are introduced into the LFD. The general mechanism is based on a limited number of label-specific binding sites in the lateral flow system. If the number of relevant labels exceeds the number of available binding sites in the lateral flow system, the overall number of signal-creating “molecular sandwiches” will be reduced (learn more about high dose hook effect). Ultimately, it is possible to calculate quite precisely which quantities of the LAMP product can be used for LFA. The following table gives an overview of hook effect-associated limitations of the HybriDetect test strips.
Table 4. Orientation for maximum amount of introduced labels into LFA
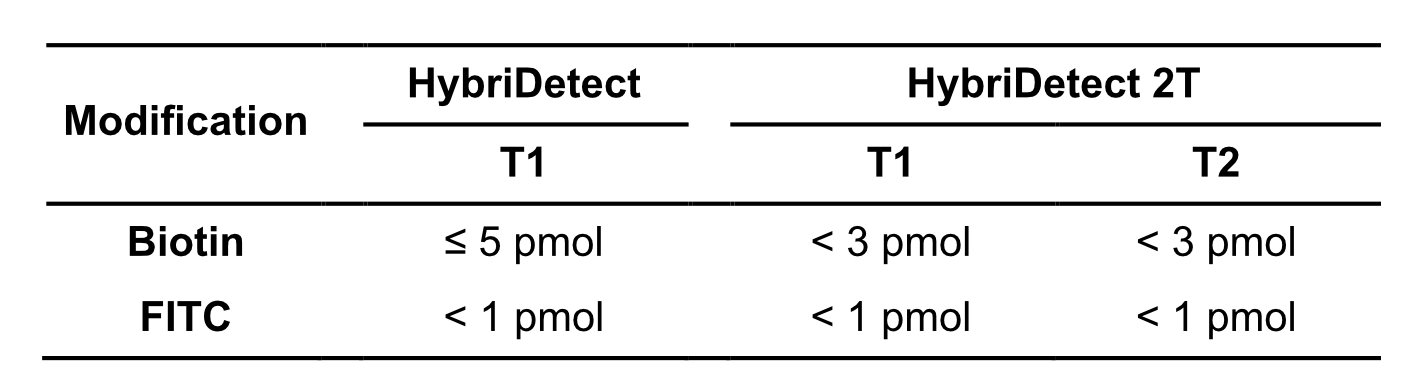
One Step RNA-Detection and Prevention of Contamination
As already mentioned, the Bst polymerase has additional reverse transcriptase activity. Furthermore LAMP reagents are perfectly compatible with available (warmstart) reverse transcriptases. However, the loop mediated isothermal amplification is a very good choice when sensitive single step detection of RNA is needed (17). This is one of the most important reasons why LAMP is coming so much into focus in the context of SARS-CoV-2 detection (18-21). It’s simplicity, speed, sensitivity, RT-compatibility combined with the diversity of amplicon detection strategies makes LAMP extremely attractive compared to other amplification techniques.
Another important feature is the compatibility of LAMP-reagents with the UDG-system (22-25). The Uracil-DNA-Glycosylase (UDG or UNG) is an enzyme, which is able to catalyze the hydrolysis of the N-glycosidic bond from deoxyuridine to release uracil from DNA. Therefore, the UDG-System (dUTP’s and UDG-restriction prior to amplification) is a frequently used tool to avoid carryover contaminations. Significantly more DNA is accumulated during a LAMP compared to PCR. This circumstance illustrates the associated risk of carryover contamination when LAMP-reactions have to be opened after amplification (more information about carryover contaminations). For this reason, it is highly recommended to consider implementing the UDG system from the beginning of LAMP-LFA development. A practical approach is the usage of dUTP-containing dNTP-Mix from the beginning. UDG can be implemented afterwards if necessary.
LAMP as part of new diagnostic detection strategies
In the current pandemic, molecular diagnostics have come into public focus as one of the most important tools in the fight against novel coronavirus-2. In this “spotlight” it was possible to direct the focus to alternative and new DNA-/ RNA-detection methods. Especially CRISPR/Cas-based detection strategies, such as SHERLOCK or DETECTR, attracted scientific and public attention. These methods are a combination of nucleic acid amplification and amplicon recognition by a CRISPR-associated protein. Interestingly these ultrasensitive and specific tests benefit from strengths of isothermal amplification. Therefore, LAMP and CRISPR/Cas-based amplicon recognition is the most popular combination to achieve a satisfying test performance. If you want to learn more about these methods, check out the following Articles from the Milenia Authors.
Three Papers you may not know, to improve your LAMP-LFA
Speed up your LAMP – Impact of Guanidine Hydrochloride (26)
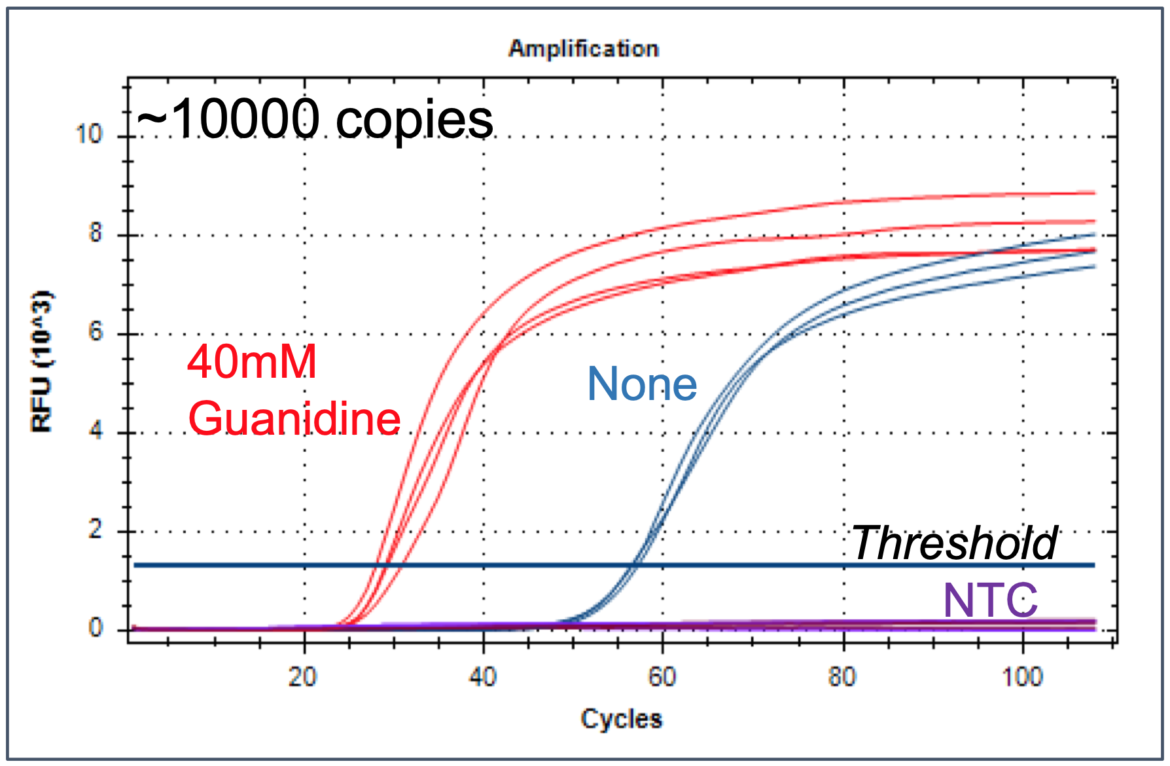
Zhang and co-workers show that the addition of guanidine hydrochloride in LAMP reactions results in a significant improvement of amplification speed and sensitivity without loss of specificity. The study presents data from SARS-CoV-detection assays with several primers sets. The speed of detection could be increased by 20 – 50% compared to reference reactions without guanidine hydrochloride. Interestingly amplification is accelerated with RNA- and DNA-templates. Moreover, another isothermal amplification method (HDA, helicase dependent amplification) can be improved by addition of guanidine hydrochloride. This preprint emphasizes the impact of LAMP-additives.
PS-LAMP – isothermal Amplification at lower temperatures (27)
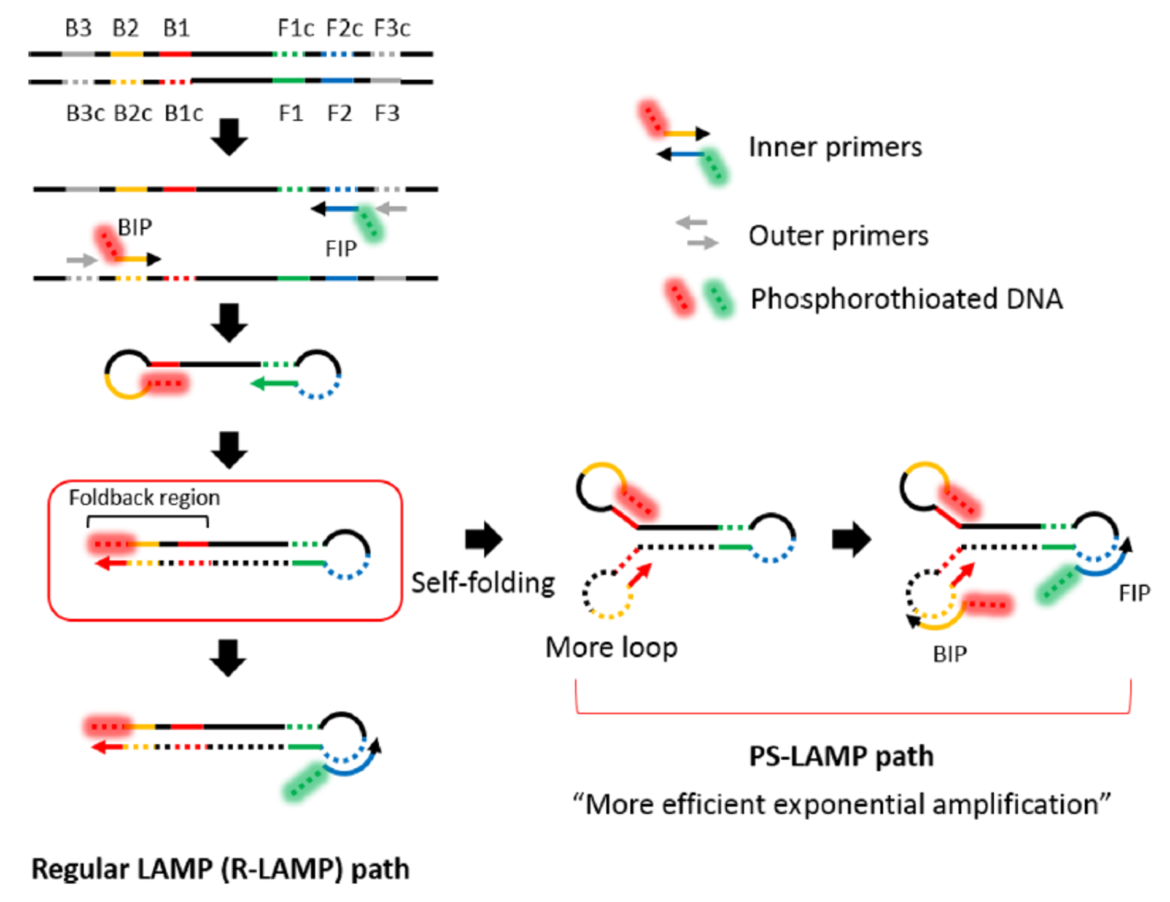
Cai and co-workers had an impressive idea of improving LAMP reaction. They tried to increase the number of loops in LAMP-amplicons in the initial phase of amplification. Therefore they used phosphorothioated primers and named their LAMP-version PS-LAMP. By increasing the number of loops, the amplification efficiency could be improved. The additional usage of chaotropes (urea) and single-stranded DNA-binding proteins (SSB) allowed sensitive and specific isothermal amplification at lower temperatures (40°C).
In LAMP-Hybridization – LFA (16)
The hybridization of LAMP-products after amplification in a separate step is a proven method in the “LAMP-LFA field” (see table 3). Jaroenram and Kampeera et al. proved functionality of their improved hybridization version.
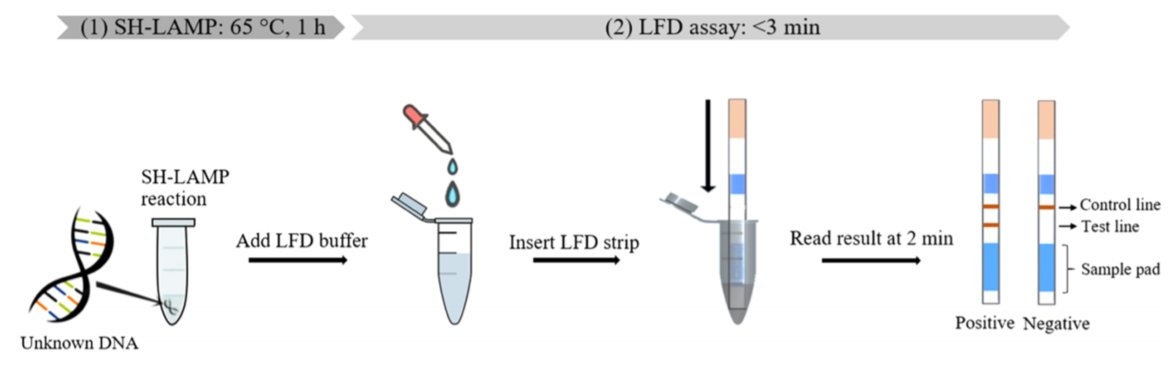
By using a 5’ FITC-labeled and 3’ elongation blocked (Spacer C3) probe, they were able to visualize LAMP products on the Milenia HybriDetect universal teststrip without an additional hybridization-step. In a practically relevant context it was shown that this alternative labeling strategy enables extremely sensitive and specific detection of Mycobacterium tuberculosis (Mtb). Although the idea of using elongation blocked probes in a LAMP is not new (15), this publication proves that the creativity in LAMP-LFA-design can result in excellent assay performance. Hopefully we’ll see the multiplex version of this detection approach very soon!
Summary
The loop mediated isothermal amplification is an impressive amplification technique. Although the development of a LAMP assay is not super easy, there are many tools and resources that can help quickly. The LAMP is suitable for very sensitive and specific (multiplex-) detection of DNA and RNA. The compatibility with the UDG-system is an important characteristic, which emphasizes the point-of-need suitability. Moreover, the method itself is continuously being optimized and there are more and more modifications of the original method, which expand the range of applications in all directions. Therefore, the LAMP is increasingly coming into public and scientific focus.
The combination of a LAMP with a Lateral Flow-based readout can result in a POC-compatible, low resource test with an excellent detection performance.
- Tomita, Norihiro et al.: “Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products.” Nature protocols 3,5 (2008): 877-82. doi:10.1038/nprot.2008.57 (2008)
- Tanner, Nathan A et al.: “Visual detection of isothermal nucleic acid amplification using pH-sensitive dyes.” BioTechniques 58,2 59-68. 1 Feb. 2015, doi:10.2144/000114253 (2015)
- Chen, Yuting et al.: “Point-of-care and visual detection of P. aeruginosa and its toxin genes by multiple LAMP and lateral flow nucleic acid biosensor.” Biosensors & bioelectronics 81 (2016): 317-323. doi:10.1016/j.bios.2016.03.006 (2016)
- Li, Jinhui et al.: “Rapid and sensitive detection of Senecavirus A by reverse transcription loop-mediated isothermal amplification combined with a lateral flow dipstick method.” PloS one 14,5 e0216245. 2 May. 2019, doi:10.1371/journal.pone.0216245 (2019)
- Yu, Jia et al.: “Improvement and evaluation of loop-mediated isothermal amplification combined with a chromatographic flow dipstick assay and utilization in detection of Vibrio cholerae.” Analytical and bioanalytical chemistry 411,3 (2019): 647-658. doi:10.1007/s00216-018-1472-1 (2019)
- Allgöwer, Stefanie M et al.: “The Development of Highly Specific and Sensitive Primers for the Detection of Potentially Allergenic Soybean (Glycine max) Using Loop-Mediated Isothermal Amplification Combined with Lateral Flow Dipstick (LAMP-LFD).” Foods (Basel, Switzerland) 9,4 423. 3 Apr. 2020, doi:10.3390/foods9040423 (2020)
- Huang, Hai-Long et al.: “Molecular method for rapid detection of the red tide dinoflagellate Karenia mikimotoi in the coastal region of Xiangshan Bay, China.” Journal of microbiological methods 168 (2020): 105801. doi:10.1016/j.mimet.2019.105801 (2020)
- Mallepaddi, Prudhvi Chand et al.: “Development of Loop-Mediated Isothermal Amplification-Based Lateral Flow Device Method for the Detection of Malaria.” The American journal of tropical medicine and hygiene 99,3 (2018): 704-708. doi:10.4269/ajtmh.18-0177 (2018)
- Liu, Dongxin et al.: “Development of a loop-mediated isothermal amplification coupled lateral flow dipstick targeting erm(41) for detection of Mycobacterium abscessus and Mycobacterium massiliense.” AMB Express 9,1 11. 23 Jan. 2019, doi:10.1186/s13568-019-0734-4 (2019)
- Wang, Yi et al.: “Detection of nucleic acids and elimination of carryover contamination by using loop-mediated isothermal amplification and antarctic thermal sensitive uracil-DNA-glycosylase in a lateral flow biosensor: application to the detection of Streptococcus pneumoniae.” Mikrochimica acta 185,4 212. 7 Mar. 2018, doi:10.1007/s00604-018-2723-8 (2018)
- Surasilp, Thanai et al.: “Rapid and sensitive detection of Vibrio vulnificus by loop-mediated isothermal amplification combined with lateral flow dipstick targeted to rpoS gene.” Molecular and cellular probes 25,4 (2011): 158-63. doi:10.1016/j.mcp.2011.04.001 (2011)
- Yongkiettrakul, Suganya et al.: “Application of loop-mediated isothermal amplification assay combined with lateral flow dipstick for detection of Plasmodium falciparum and Plasmodium vivax.” Parasitology international 63,6 (2014): 777-84. doi:10.1016/j.parint.2014.06.004 (2014)
- Kumvongpin, Ratchanida et al.: “Detection assay for HPV16 and HPV18 by loop‑mediated isothermal amplification with lateral flow dipstick tests.” Molecular medicine reports 15,5 (2017): 3203-3209. doi:10.3892/mmr.2017.6370 (2017)
- Khunthong, Sasiwarat et al.: “Rapid and sensitive detection of shrimp yellow head virus by loop-mediated isothermal amplification combined with a lateral flow dipstick.” Journal of virological methods 188,1-2 (2013): 51-6. doi:10.1016/j.jviromet.2012.11.041 (2013)
- Phillips, Elizabeth A et al.: “Strand Displacement Probes Combined with Isothermal Nucleic Acid Amplification for Instrument-Free Detection from Complex Samples.” Analytical chemistry 90,11 (2018): 6580-6586. doi:10.1021/acs.analchem.8b00269 (2018)
- Jaroenram, Wansadaj et al.: “Ultrasensitive detection of Mycobacterium tuberculosis by a rapid and specific probe-triggered one-step, simultaneous DNA hybridization and isothermal amplification combined with a lateral flow dipstick.” Scientific reports 10,1 16976. 12 Oct. 2020, doi:10.1038/s41598-020-73981-6 (2020)
- Arunrut, Narong et al.: “Rapid and sensitive detection of Laem-Singh virus by reverse transcription loop-mediated isothermal amplification combined with a lateral flow dipstick.” Journal of virological methods 177,1 (2011): 71-4. doi:10.1016/j.jviromet.2011.06.020 (2011)
- Lau, Yee Ling et al.: “A Sensitive Reverse Transcription Loop-Mediated Isothermal Amplification Assay for Direct Visual Detection of SARS-CoV-2.” The American journal of tropical medicine and hygiene, 10.4269/ajtmh.20-1079. 23 Oct. 2020, doi:4269/ajtmh.20-1079 (2020)
- Javalkote, Vivek S et al.: “CRISPR-based assays for rapid detection of SARS-CoV-2.” Methods (San Diego, Calif.), S1046-2023(20)30217-6. 9 Oct. 2020, doi:1016/j.ymeth.2020.10.003 (2020)
- Brandsma, Eelke et al.: “Rapid, sensitive and specific SARS coronavirus-2 detection: a multi-center comparison between standard qRT-PCR and CRISPR based DETECTR.” The Journal of infectious diseases, jiaa641. 10 Oct. 2020, doi:1093/infdis/jiaa641 (2020)
- Mohon, Abu Naser et al.: “Optimization and clinical validation of dual-target RT-LAMP for SARS-CoV-2.” Journal of virological methods, vol. 286 113972. 15 Sep. 2020, doi:1016/j.jviromet.2020.113972 (2020)
- Manajit, Orapan et al.: “Development of uracil-DNA-glycosylase-supplemented loop-mediated isothermal amplification coupled with nanogold probe (UDG-LAMP-AuNP) for specific detection of Pseudomonas aeruginosa.” Molecular medicine reports 17,4 (2018): 5734-5743. doi:10.3892/mmr.2018.8557 (2018)
- Wang, Yi et al.: “Loop-mediated isothermal amplification using self-avoiding molecular recognition systems and antarctic thermal sensitive uracil-DNA-glycosylase for detection of nucleic acid with prevention of carryover contamination.” Analytica chimica acta 996 (2017): 74-87. doi:10.1016/j.aca.2017.10.022 (2017)
- Wang, Yi et al.: “Detection of nucleic acids and elimination of carryover contamination by using loop-mediated isothermal amplification and antarctic thermal sensitive uracil-DNA-glycosylase in a lateral flow biosensor: application to the detection of Streptococcus pneumoniae.” Mikrochimica acta 185,4 212. 7 Mar. 2018, doi:10.1007/s00604-018-2723-8 (2018)
- Hsieh, Kuangwen et al.: “Simultaneous elimination of carryover contamination and detection of DNA with uracil-DNA-glycosylase-supplemented loop-mediated isothermal amplification (UDG-LAMP).” Chemical communications (Cambridge, England) 50,28 (2014): 3747-9. doi:10.1039/c4cc00540f (2014)
- Zhang, Y. et al.: “Enhancing LAMP Amplification Speed and Sensitivity with Guanidine Chloride” bioRxiv 2020.06.03.132894; doi: https://doi.org/10.1101/2020.06.03.132894 ()
- Cai, Sheng et al.: “Phosphorothioated Primers Lead to Loop-Mediated Isothermal Amplification at Low Temperatures.” Analytical chemistry 90,14 (2018): 8290-8294. doi:10.1021/acs.analchem.8b02062 (2018)



