The SHERLOCK method was continously improved in the last few years to make specific POC tests possible:
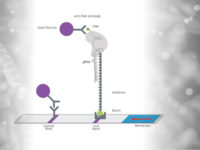
What is HybriDetect?
The HybriDetect is a lateral flow dipstick (LFD), which is capable to detect different molecules, including gene amplification products, proteins and antibodies. A commonly used application for our test is the detection of gene amplification products resulting from PCR, LAMP or RPA. The underlying genetic information can be detected regardless which kind of organism need to be detected. Finally, labeled primers must be introduced in the amplification step, so that the resulting fragments are labeled and can be detected by the HybriDetect dipstick.
Results can be reported within 5 minutes after lines become visible on the test strip.
If you want to learn more about Milenia HybriDetect, see our other blog articles:
COVID-19 Rapid Test – Detection Methods
At the moment, the standard COVID 19 test is a qRT-PCR (quantitative real time polymerase chain reaction). Obviously, there are several reasons why qRT-PCR test cannot be used as a POCT (point of care test). First of all, it takes several hours to do a qPCR test, second you need highly trained personnel and third special equipment is essential. In the current situation, with the entire world dealing with the COVID-19 outbreak, it is of great interest to develop rapid tests for POC testing of the new corona virus to help people in sparsely populated areas where laboratories are difficult to reach.
Since the outbreak of COVID-19 at the end of 2019 several tests for SARS-CoV-2 detection have been developed and approved. But most of them show several disadvantages:
- Expensive laboratory equipment is needed
- High level technical expertise is required
- Access to reagents is difficult
- Expensive
With the currently available rapid Antigen tests, a result is available after 15 minutes outside the lab. But the Antigen tests are detecting viral proteins, and are therefore less sensitive as a test on DNA level.
Due to these reasons, several groups are working on alternative tests for the quick, easy and low cost detection of SARS-CoV-2 with very little equipment needed.
Why do we need more rapid tests?
The head of the WHO Tedros Adhanom Ghebreyesus already told on March 16th, that there has not been an urgent enough escalation in testing. His key message on this day was: test, test, test.
The most important point is to detect local outbreaks as soon as possible and control the spread of the COVID-19 virus through quarantine measures. The effect of the high test capacities could be seen in South Korea. They rely on early PCR tests to record lots of cases, even mild and symptom free ones. Tests are offered in drive troughs and walk troughs. Consequently, the number of infections in South Korea stayed relatively low.
At a school in Mecklenburg-West Pomerania, in Germany all teachers and students, even their parents are offered free tests to identify and contain infections early. The tests are voluntary and offered twice a week. Mecklenburg-West Pomerania is planning to do the COVID-19 tests in every school, to get back to “normal life conditions” quickly.
These two examples show the high need for frequent testing. Obviously, obtaining fast test results is one of the biggest advantages of point-of-care compatible rapid tests. Generating quick test results without the need of sending the samples to an external laboratory allows quick reaction and can help to control local outbreaks.
Here we give a short overview of the latest publications dealing with the rapid detection of COVID-19 combined with the HybriDetect Lateral Flow Method for visualization.
COVID 19 testing and CRISPR
Promising testing methods, which are based on isothermal amplification in combination with a CRISPR-mediated detection (SHERLOCK (1), DETECTR (2)) have been developed.
SHERLOCK
The group of the inventor of the SHERLOCK method Feng Zhang and collegues, based in the United States, described a method for COVID-19 detection using CRISPR. The scientists were able to detect synthetic COVID-19 virus RNA fragments between 20 and 200 aM (10-100 copies per µl of input). They used purified RNA as Input for an RT-RPA before the Cas13 assay. The whole method takes less than one hour.
The following picture shows an example of the COVID-19 detection using our HybriDetect.
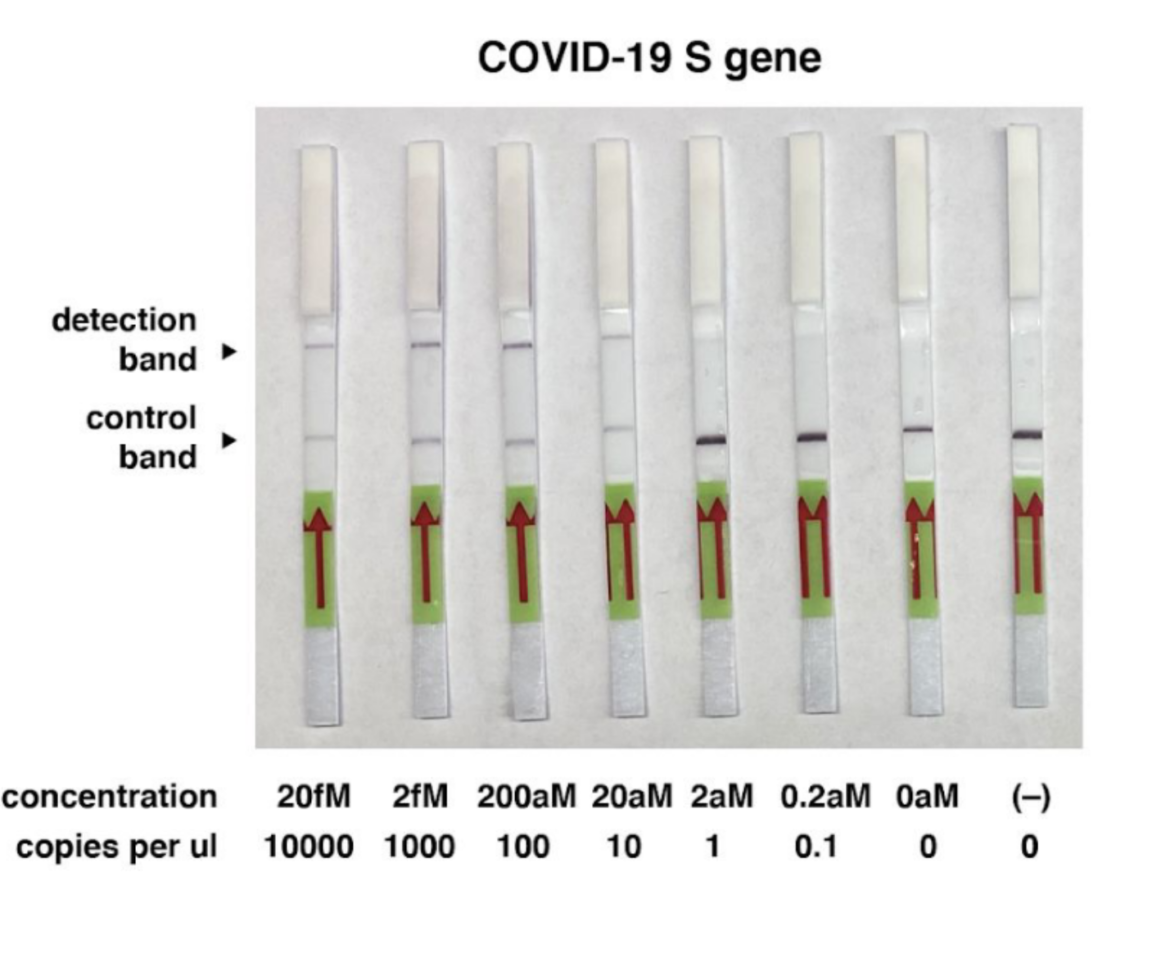
- For more information see the original paper.
- Zhang et al give a precise description, how the SHERLOCK technique is done in nature protocol:
STOPCovid – improved SHERLOCK method
Due to the fact, that the method described above needs two reaction steps (isothermal amplification and CRISPR-reaction) it is still complex and cross-contamination in an uncontrolled environment can happen quickly. For these reasons, Zhang and colleagues recently published an update (preprint, not peer reviewed) on the SHERLOCK method, which can be performed in one step (3) and is a promising rapid COVID 19 detection method.
- 2016: Detection of Cas13 by Zhang lab
- 2018: Improvement of the Cas enzymes Cas12b and Cas13 (SHERLOCKv2)
- 2019: First paper on the specific detection of DNA and RNA through CRISPR-Cas from clinical relevant samples. Step-by-step instructions with RPA
- 2020: STOPCovid: One Pot reaction combines LAMP and SHERLOCK to detect Sars-CoV-2
The so-called STOP (SHERLOCK Testing in One Pot) method is an improvement of the previous SHERLOCK application. This test allows a turnaround time of an hour from sampling to the report of the resuls. The very simple handling underlines the potential as a point-of-care test (POCT) for COVID 19 testing.
General information about STOP:
- Results in 40 to 70 minutes
- Detection of 100 copies of viral genome
- Saliva or nasopharyngeal swabs as input
- Specific detection
- Single temperature, one fluid handling step, visual readout
- Nearly no equipment
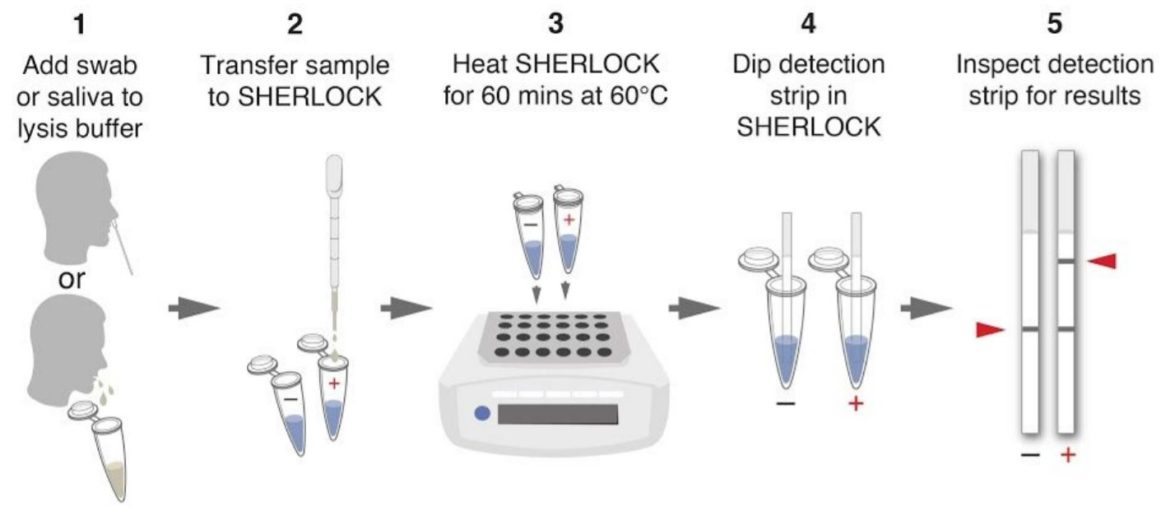
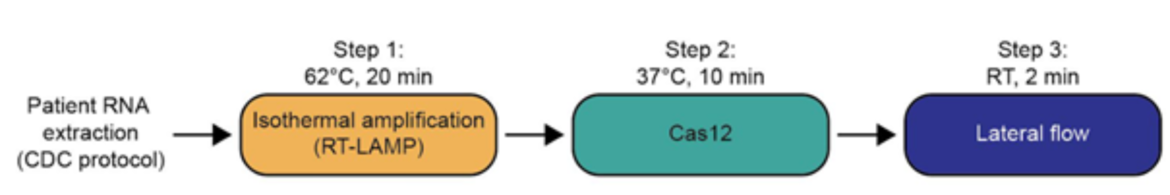
One pot reaction in three simple steps
- Step 1: 10 mins at 22°C or at 60°C → lysis of virus-containing patient sample (add Proteinase K inhibitor) or 5 mins at 95°C
- Step 2: 1 hr at 60°C → detection of viral RNA using STOPCovid reaction
- Step 3: 2 mins at 22°C → visual read out of the detection results by eye using HybriDetect
If you want to learn more about the lateral flow readout of HybriDetect in combination with CRISPR, read our related article: Lateral Flow Readout for CRISPR/Cas-based detection strategies
Combining RT-LAMP, Cas12b and HybriDetect
The authors used a RT-LAMP (loop-mediated isothermal amplification) for RNA-transcription followed by simple isothermal DNA-amplification, which works at 55-65°C. For this reason they needed a thermostable Cas-Protein, which is active at 60°C. They found Cas12b from Alicyclobacillus acidiphilus (Aap) to be stable at this temperature. Out of 29 primer sets for detecting SARS-CoV-2 the scientists found LAMP-compatible primer sets. The next step was to find the right guide RNA (gRNA), which was figured out comparing 18 gRNAs.
During another optimization it was discovered that the addition of taurin resulted in an improvement of the reaction kinetics. After RT-LAMP and CRISPR-reaction (Step 3 in Figure 2) HybriDetect can be dipped directly into the reaction tube at room temperature and visual read out can be done after 2 minutes.
The Authors provide all the information needed to do the STOPCovid test in their paper: LAMP Primers, gRNA sequence, Cas12b-Protein Sequence, etc.
Validation of STOPCovid
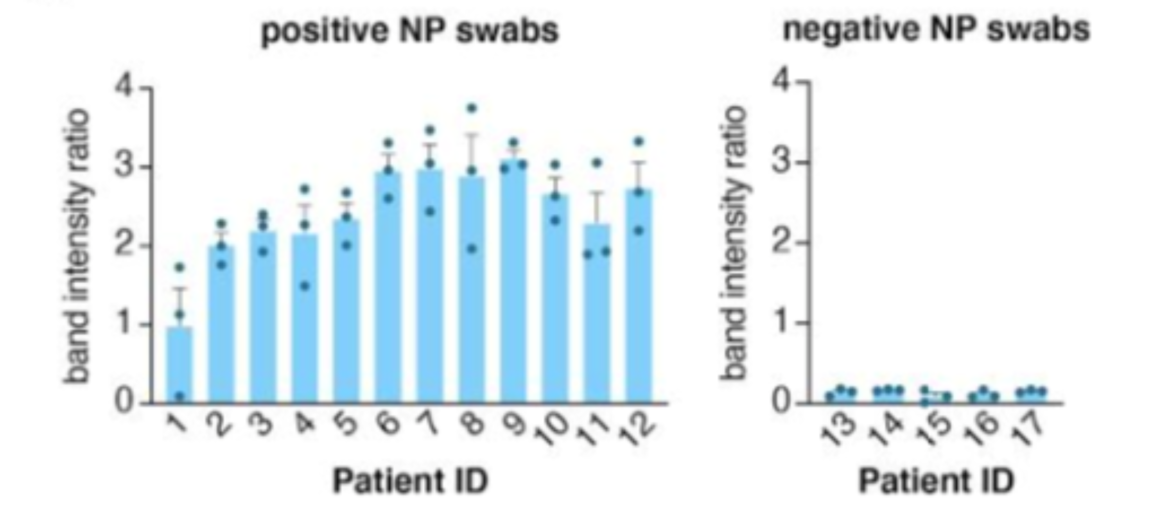
The method was validated on 17 patient samples. It could detect 12 positive and 5 negative samples, 2 of 3 replicates were scored positive in infected patients. STOPCovid was able to detect all positive and all negative samples (Fig. 3) compared to the gold standard method RT-qPCR.
STOPCovid.v2
The inventors of STOPCovid are constantly improving the Covid-19 detection method for rapid testing. In theire latest update on the method they described a magnetic bead purification method. With this approach, they could reduce the sample extraction time to 15 minutes. (Fig. 5)
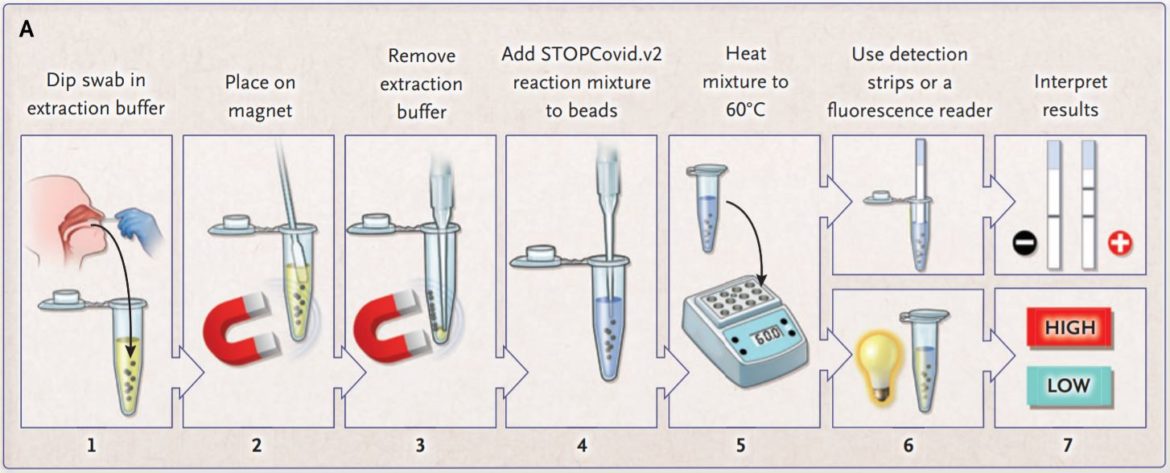
The researchers compared the STOPCovid.v2 with the gold standard RT-qPCR. The result was a reduction of viral RNA that was 600 times of the input needed for the RT-qPCR. The STOPCovid.v2 has a limit of detection (LOD) similar to a cycle-theshold (Ct) value of 40.3. StopCovid.v2 was tested on 202 SARS-CoV-2 positive and 200 SARS-CoV-2 negative samples. The test result showed a sensitivity of 93.1% and a specificity of 98.5%. The positive samples were detected in 15 to 45 minutes.
Read the original article here
What is the clue about the updated version of SHERLOCK?
Taken together the striking advantage of this method is its simplicity. The 3 steps, shown above are very easy to do, even for untrained personnel. The authors even showed that saliva samples worked as input for the reaction. This makes the method performable for lay users. Another advantage is the low-tech equipment approach, which has a major impact on the POC-compatibility.
Scientists who are interested in testing the protocol can get more information and test kits at STOPCovid.science.
The protocol is not authorized by the FDA (food and drug administration), it is not for clinical purposes.
Read the original article here.
DETECTR
Broughton et al. already published a paper, how to detect SARS_CoV-2 using LAMP, Cas12 and our HybriDetect within 30 minutes. They are using isothermal pre-amplification with primers published by the WHO and CDC. As the authors mentioned in the paper, this application would be a helpful tool for POCT testing in emergency departments, airports etc..
For more information see the original paper.
Here you can find a video from the McGovern Institute describing the SHERLOCK technique in combination with the HybriDetect in General!
In summary, rapid testing is being expanded to stop the spread of Covid 19. Scientists in the United States and India (read more about the Feluda Test) developed new tests on Genome level, which are nearly as fast as rapid antigen tests, but more sensitive.
With these new approaches, departments of health could be relieved and the public health improved.
- Zhang et al: A protocol for detection of COVID-19 using CRISPR diagnostics. (2020)
- Broughton et al: CRISPR–Cas12-based detection of SARS-CoV-2. (2020)
- Joung et al.: Point-of-care testing for COVID-19 using SHERLOCK diagnostics (2020)