„Milenia HybriDetect connects sensitive and specific, molecular applications with the lateral flow technology. The combination makes it possible to discover an entirely new generation of point-of-care diagnostics.“

Milenia HybriDetect – Types of Use
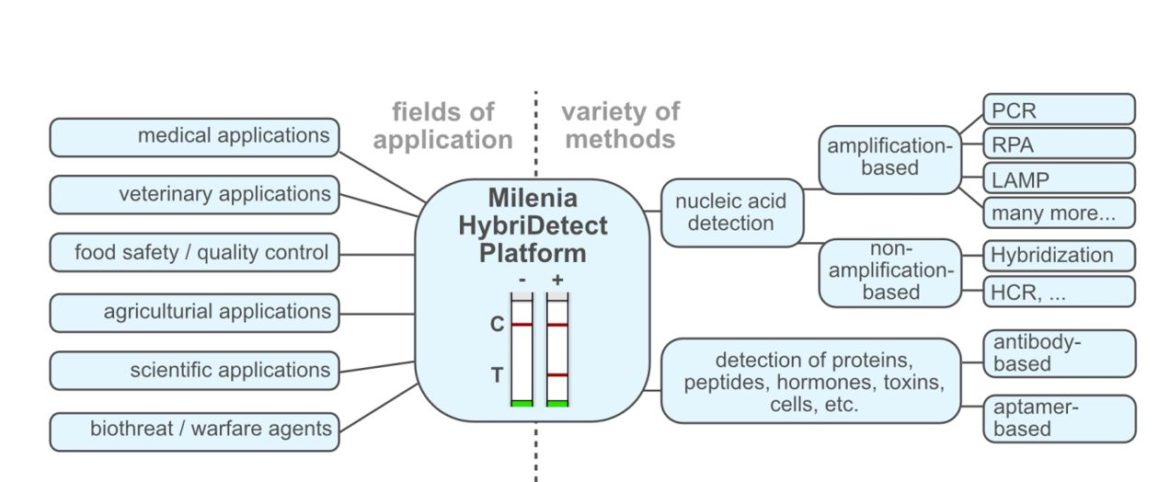
The universal test strips can be combined with multiple methods. Up to now, one interesting detail has always been left out of this consideration. The lateral flow devices can be used in a variety of creative ways. As dipsticks, the strips can simply be placed directly into reaction vessels. in cassette format, the LFDs can be attractive for commercial applications. But it is possible to be much more creative – the HybriDetect test strips have already been used in wearable diagnostics or implemented in more complex devices such as a rotating disc…
Dipsticks
The Milenia HybriDetect products are intended as tools for simple development of innovative rapid test concepts. The use of molecular biological methods (e.g. DNA amplification) is undoubtedly at the center of attention. Therefore, standardized consumables such as 200 µL PCR tubes are generally used. These are easy to handle, always available and can be combined with many instruments. The dimensions of the Milenia HybriDetect dipsticks allow simple dipping of the strips in such reaction tubes. The following picture shows several dipsticks placed in PCR strips including a sample volume of 50 µL. Clear signals are detectable within 2-5 minutes. In addition to PCR tubes, 96-well plates, 1.5 mL tubes or other reaction vessels can also be used. The dipsticks are extremely versatile. In addition, this lateral flow format provides further important advantages which will be discussed in more detail elsewhere. From our point of view the dipsticks are the perfect format for easy and fast development of innovative rapid tests.

Lateral Flow Cassettes
The most common way to use the lateral flow technology is the usage of plastic cassettes. This has some advantages for the usability. It simplifies sample application, protects the sensitive parts of the device and thus helps to find test- and control lines. However, the use of this plastic housing requires more material, space and production effort. Currently, we do not offer such a version of the Milenia HybriDetect. Nevertheless, we do not categorically exclude this. For individual requests, it is certainly possible to obtain the Milenia HybriDetect lateral flow devices in cassette format. We would be happy to discuss this on an individual level and can address the advantages and disadvantages with all our experience.
Implementation into other Devices
The dipsticks can also be the basis for further development of alternative, innovative and more complex devices for „sample in – result out“ analytics. A good example is the paper-based, foldable detection system that includes the Milenia HybriDetect. Cordray and Richards-Cortum published in 2015 a simple, innovative and field-applicable system that allows the sensitive detection of Plasmodium DNA (10, malaria diagnostics).
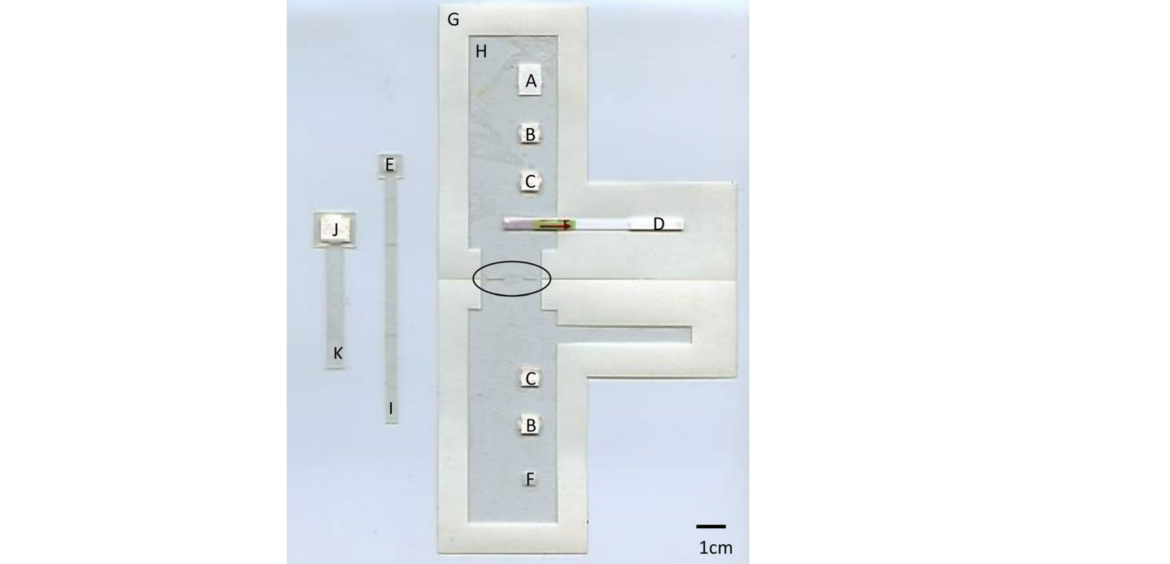
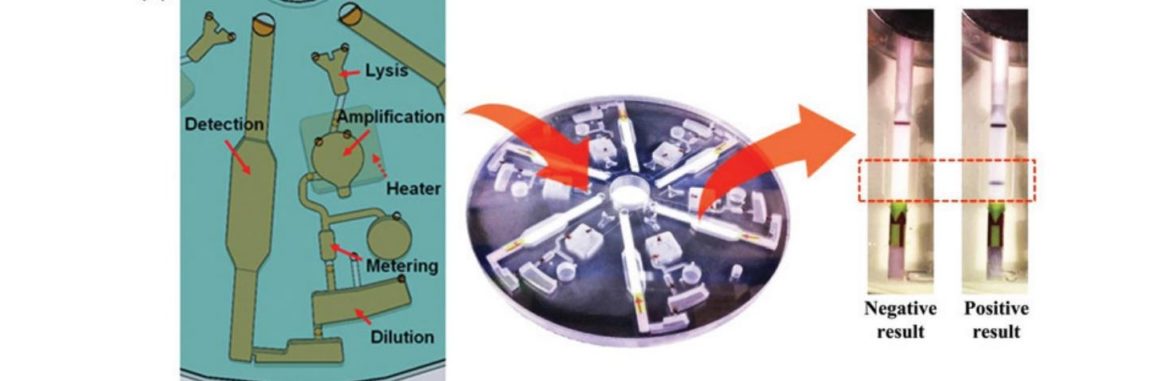
In 2014, Kim et al. presented an entire analysis platform for the nucleic acid-based analysis of food-borne pathogens (54). Here, 6 Milenia HybriDetect test strips were implemented in a rotating disc system. In a combination of RPA and lateral flow, multiplexing is achieved by using this system, which outputs multiple information per analysis. If you want to learn more about Multiplexing and Lateral Flow, see one of our previous articles: Milenia HybriDetect – Lateral Flow and Multiplexing
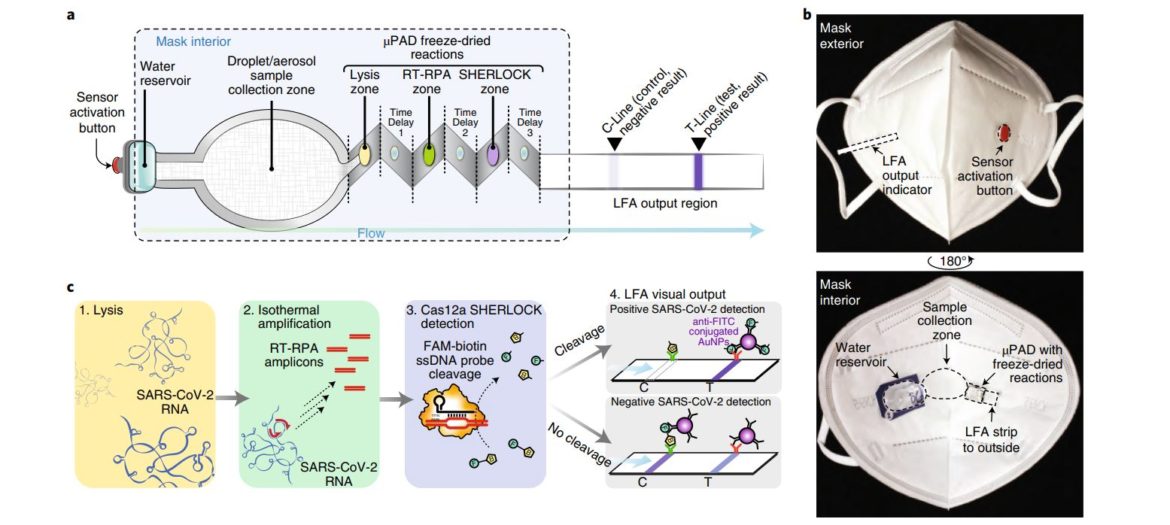
Implementation into Face Mask
One of the most creative ways to use our lateral flow dipsticks was presented by authors of the Broad Institute in Boston around Peter Nguyen. They have converted a face mask into a valuable diagnostic tool to detect SARS-CoV-2 as sensitive as a lab-based technique. Aerosols are collected in a sample collection zone. Detection reagents come into contact with the sample matrix via a sensor activation button. Reverse transcription, isothermal recombinase polymerase amplification, Cas12a-dependent amplicon recognition and degradation of a dual-labeled reporter molecule are combined in on device. Finally, the sample is transferred to the dipstick and the result can be interpreted within minutes. That is impressive!
Fields of Application
The Milenia HybriDetect is used wherever rapid tests make sense. Because of this, the Milenia HybriDetect is used in a wide variety of relevant areas. In addition to medical and veterinary applications, this includes sectors such as agriculture, food safety, quality control and many more. The following list includes the most important sectors in which the Milenia HybriDetect is used.
Medical Applications
Rapid testing has always been an important facette of medical diagnostics, because time matters. The Milenia HybriDetect connects sensitive and specific, molecular applications with the lateral flow technology. The combination makes it possible to discover an entirely new generation of point-of-care diagnostics. Intra-operative analyses that produce relevant results within minutes and influence the progress of the operation. Molecular bediside diagnostics quickly deliver results that previously required a specialized laboratory, trained personnel and, most importantly, a lot of time. Individualized diagnostics are also within reach, thanks to the modular, universal nature of the Milenia HybriDetect platform. The following point underscrore the value and potential of such medical applications …
- post transplant infections (19)
- periprosthetic joint infections (17)
- detection of viral pathogens: SARS-CoV (9, 18, 35), Zika Virus (28), Dengue Virus (28), Human Papilloma Viruses (43), Eppstein-Barr Virus, Ebola Virus (16) and Lassa Virus (7), human Adenovirus (36)
- detection of bacterial pathogens: MRSA (30, 41), Mycobacterium tuberculosis (2), Ricksettsia ricksettsii (34), Borrelia (25), Group B Streptococcus(15)
- detection of pathogenic yeast: Cyptococcus (27)
- detection of pathogenic other parasites: Malaria diagnostics – Plasmodium (10, 23, 44), Cryptosporidium (11), Toxoplasma gondii (21), Entamoeba histolytica (29), Leishmania viannia spp. (38), etc.
- SNP-genotyping (45)
- cancer-related biomarkers (14)
Veterinary and ecological Applications
Especially in the veterinary context, diagnostic tools for point-of-need applications play an important role. The connection to molecular biology opens up new possibilities for veterinarians. Monitoring and detection of animal diseases in the field are of particular importance in this context.
- species identification / ecological studies (51)
- detection of animal pathogens – epidemic agents:
- fish viruses (24 ,26 ,40)
- shrimp viruses (20),
- African Swine Fever Virus (6, 48),
- Porcine Parvovirus ,
- Porcine Circovirus Type 2,
- Bovine Leukemia Virus,
- Mycoplasma bovis (50), etc.
Food Safety / Quality Control
The food safety sector can also benefit massively from innovative, molecular, point-of-need compatible methods. Milenia Biotec GmbH has developed its own detection systems based on Milenia HybriDetect, which play a role in the beverage industry. During this process, we were able to experience that a simplification of existing technologies such as pcr can bring great benefits for microbiological analysis. Consequently, we can tell from our own experience that there is a great need for simple, accurate and sensitive techniques in this field of application.
- hygienic control (5) surface monitoring in breweries (Milenia GenLine direct detection of beer spoilage bacteria from swabs)
- foodborne pathogens: Salmonella (13) , Listeria spp., Listeria monocytogenes (22), etc.
- beverage spoiling organisms: water contaminants, beer spoiling bacteria, fruit juice contaminants, etc.
- allergen detection (3)
- GMO-monitoring
- product authentification (42)
Agriculture
It is obvious that simple molecular analytics that can be applied in the field will be of great relevance, especially in the agricultural sector. Phytopathological questions are of particular importance. But also GMO monitoring and authentication are relevant thematic aspects that can be addressed here.
- plant health
- GMO monitoring
- Species identification
- plant monitoring (1)
- plant pathogen detection
- fungal plant pathogens: Macrophomia phaesolina (31), Phytophtora species (12, 46)
- plant viruses (49)
- nematodes: Meloidogyne hapla (32)
- bacterial plant pathogens: Clavibacter michiganenensis (37)
Biothreat / Warfare Agents
This is a very special topic, where the speed and accuracy of the analysis are of decisive importance. Simple, direct and rapid molecular analyses can be essential tools for assessing such situations. Of course, it would be best, if such tests were not needed at all.
- Bacillus anhtracis, Burkholderia pseudomallei (33, 39), Coxiella burnetii, etc.
From the Idea to a Potential Product
The use of an universal LFD has important advantages that should be considered at the very beginning of assay development. The universal character of the Milenia HybriDetect allows fast assay development, because the LFD in the test system works reliably, consistently and is available in both, large and small quantities. Moreover, the test system is easily adaptable for additional applications. Should a test system work properly and inquiries regarding commercialization increase, the Milenia HybriDetect can represent a safe and constantly available component from the very beginning of the project. Ultimately, this platform offers numerous advantages, ranging from the initial idea to a potential commercial product.
If you want to learn about the Milenia HybriDetect & Milenia HybriDetect 2T, you are welcome to check out the following content:
- : Abudayyeh, O. O., Gootenberg, J. S., Kellner, M. J., & Zhang, F. (2019). Nucleic Acid Detection of Plant Genes Using CRISPR-Cas13. The CRISPR Journal, 2(3), 165–171. https://doi.org/10.1089/crispr.2019.0011 ()
- : Ağel, E., Sağcan, H., Ceyhan, İ., & Durmaz, R. (2020). Optimization of isothermal amplification method for Mycobacterium tuberculosis detection and visualization method for fieldwork. Turkish Journal of Medical Sciences, 50(4), 1069–1075. https://doi.org/10.3906/sag-1910-6 ()
- : Allgöwer, S. M., Hartmann, C. A., & Holzhauser, T. (2020). The development of highly specific and sensitive primers for the detection of potentially allergenic soybean (Glycine max) using loop-mediated isothermal amplification combined with lateral flow dipstick (LAMP-LFD). Foods, 9(4). https://doi.org/10.3390/foods9040423 ()
- : Azhar, M., Phutela, R., Kumar, M., Hussain Ansari, A., Rauthan, R., Gulati, S., … Maiti, S. (2020). Rapid, accurate, nucleobase detection using FnCas9 Single sentence summary A method to identify nucleotide sequence or nucleobase identity using FnCas9 and its implementation in the rapid and accurate diagnosis of SARS-CoV-2. MedRxiv, 2020.09.13.20193581. https://doi.org/10.1101/2020.09.13.20193581 ()
- : Azinheiro, S., Carvalho, J., Prado, M., & Garrido-Maestu, A. (2020). Application of recombinase polymerase amplification with lateral flow for a naked-eye detection of listeria monocytogenes on food processing surfaces. Foods, 9(9). https://doi.org/10.3390/foods9091249 ()
- : Bai, J., Lin, H., Li, H., Zhou, Y., Liu, J., Zhong, G., … Huang, L. (2019). Cas12a-Based On-Site and Rapid Nucleic Acid Detection of African Swine Fever. Frontiers in Microbiology, 10. https://doi.org/10.3389/fmicb.2019.02830 ()
- : Barnes, K. G., Lachenauer, A. E., Nitido, A., Siddiqui, S., Gross, R., Beitzel, B., … Sabeti, P. C. (2020). Deployable CRISPR-Cas13a diagnostic tools to detect and report Ebola and Lassa virus cases in real-time. Nature Communications, 11(1). https://doi.org/10.1038/s41467-020-17994-9 ()
- : Breitbach A., Jacob F., Hutzler M., Koob J. (2017). Simple Rapid Test for Establishing the Presence of Beer-Spoilage Organisms. Brauwelt International. Link to article. ()
- : Broughton, J. P., Deng, X., Yu, G., Fasching, C. L., Servellita, V., Singh, J., … Chiu, C. Y. (2020). CRISPR–Cas12-based detection of SARS-CoV-2. Nature Biotechnology, 38(7), 870–874. https://doi.org/10.1038/s41587-020-0513-4 ()
- : Cordray, M. S., & Richards-Kortum, R. R. (2015). A paper and plastic device for the combined isothermal amplification and lateral flow detection of Plasmodium DNA. Malaria Journal, 14(1). https://doi.org/10.1186/s12936-015-0995-6 ()
- : Crannell, Z. A., Castellanos-Gonzalez, A., Irani, A., Rohrman, B., White, A. C., & Richards-Kortum, R. (2014). Nucleic acid test to diagnose cryptosporidiosis: Lab assessment in animal and patient specimens. Analytical Chemistry, 86(5), 2565–2571. https://doi.org/10.1021/ac403750z ()
- : Dai, T., Hu, T., Yang, X., Shen, D., Jiao, B., Tian, W., & Xu, Y. (2019). A recombinase polymerase amplificationlateral flow dipstick assay for rapid detection of the quarantine citrus pathogen in China, Phytophthora hibernalis. PeerJ, 2019(11). https://doi.org/10.7717/peerj.8083 ()
- : Gao, W., Huang, H., Zhu, P., Yan, X., Fan, J., Jiang, J., & Xu, J. (2018). Recombinase polymerase amplification combined with lateral flow dipstick for equipment-free detection of Salmonella in shellfish. Bioprocess and Biosystems Engineering, 41(5), 603–611. https://doi.org/10.1007/s00449-018-1895-2 ()
- : Hao, L., Zhao, R., Ngambenjawong, C., Fleming, H., & Bhatia, S. (2020). CRISPR-Cas-amplified urine biomarkers for multiplexed and portable cancer diagnostics. BioRxiv. https://doi.org/10.1101/2020.06.17.157180 ()
- : Hu, S., Zhong, H., Huang, W., Zhan, W., Yang, X., Tang, B., … Luo, M. (2019). Rapid and visual detection of Group B streptococcus using recombinase polymerase amplification combined with lateral flow strips. Diagnostic Microbiology and Infectious Disease, 93(1), 9–13. https://doi.org/10.1016/j.diagmicrobio.2018.07.011 ()
- : James, A. S., Todd, S., Pollak, N. M., Marsh, G. A., & Macdonald, J. (2018). Ebolavirus diagnosis made simple, comparable and faster than molecular detection methods: Preparing for the future. Virology Journal, 15(1). https://doi.org/10.1186/s12985-018-0985-8 ()
- : Janz, V., Schoon, J., Morgenstern, C., Preininger, B., Reinke, S., Duda, G., Breitbach, A., Perka, C. F., & Geissler, S. (2018). Rapid detection of periprosthetic joint infection using a combination of 16s rDNA polymerase chain reaction and lateral flow immunoassay: A Pilot Study. Bone & joint research, 7(1), 12–19. https://doi.org/10.1302/2046-3758.71.BJR-2017-0103.R2 ()
- : Joung, J., Ladha, A., Saito, M., Kim, N.-G., Woolley, A. E., Segel, M., … Zhang, F. (2020). Detection of SARS-CoV-2 with SHERLOCK One-Pot Testing. New England Journal of Medicine, 383(15), 1492–1494. https://doi.org/10.1056/nejmc2026172 ()
- : Kaminski, M. M., Alcantar, M. A., Lape, I. T., Greensmith, R., Huske, A. C., Valeri, J. A., … Collins, J. J. (2020). A CRISPR-based assay for the detection of opportunistic infections post-transplantation and for the monitoring of transplant rejection. Nature Biomedical Engineering, 4(6), 601–609. https://doi.org/10.1038/s41551-020-0546-5 ()
- : Kusumawati, A., Tampubolon, I. D., Hendarta, N. Y., Salasia, S. I. O., Wanahari, T. A., Mappakaya, B. A., & Hartati, S. (2015). Use of reverse transcription loop-mediated isothermal amplification combined with lateral flow dipstick for an easy and rapid detection of Jembrana disease virus. VirusDisease, 26(3), 189–195. https://doi.org/10.1007/s13337-015-0277-5 ()
- : Lalle, M., Possenti, A., Dubey, J. P., & Pozio, E. (2018). Loop-Mediated Isothermal Amplification-Lateral-Flow Dipstick (LAMP-LFD) to detect Toxoplasma gondii oocyst in ready-to-eat salad. Food Microbiology, 70, 137–142. https://doi.org/10.1016/j.fm.2017.10.001 ()
- : Ledlod, S., Bunroddith, K., Areekit, S., Santiwatanakul, S., & Chansiri, K. (2020). Development of a duplex lateral flow dipstick test for the detection and differentiation of Listeria spp. and Listeria monocytogenes in meat products based on loop-mediated isothermal amplification. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, 1139. https://doi.org/10.1016/j.jchromb.2019.121834 ()
- : Lee, R. A., De Puig, H., Nguyen, P. Q., Angenent-Mari, N. M., Donghia, N. M., McGee, J. P., … Collins, J. J. (2020). Ultrasensitive CRISPR-based diagnostic for field-applicable detection of Plasmodium species in symptomatic and asymptomatic malaria. Proceedings of the National Academy of Sciences of the United States of America, 117(41), 25722–25731. https://doi.org/10.1073/pnas.2010196117 ()
- : Liu, L., Xu, Y., Zhong, W., Li, L., Li, W., & Xiao, Q. (2019). Comparison of three terminal detection methods based on loop mediated isothermal amplification (Lamp) assay for spring viremia of carp virus (svcv). Turkish Journal of Fisheries and Aquatic Sciences, 19(9), 805–816. https://doi.org/10.4194/1303-2712-v19_9_09 ()
- : Liu, W., Liu, H. X., Zhang, L., Hou, X. X., Wan, K. L., & Hao, Q. (2016). A novel isothermal assay of Borrelia burgdorferi by recombinase polymerase amplification with lateral flow detection. International Journal of Molecular Sciences, 17(8). https://doi.org/10.3390/ijms17081250 ()
- : Loose, F. N., Breitbach, A., Bertalan, I., Rüster, D., Truyen, U., & Speck, S. (2020). Diagnostic validation of a rapid and field-applicable PCR-lateral flow test system for point-of-care detection of cyprinid herpesvirus 3 (CyHV-3). PLoS ONE, 15(10 October). https://doi.org/10.1371/journal.pone.0241420 ()
- : Ma, Q., Yao, J., Yuan, S., Liu, H., Wei, N., Zhang, J., & Shan, W. (2019). Development of a lateral flow recombinase polymerase amplification assay for rapid and visual detection of Cryptococcus neoformans/C. gattii in cerebral spinal fluid. BMC Infectious Diseases, 19(1), 1–9. https://doi.org/10.1186/s12879-019-3744-6 ()
- : Myhrvold, C., Freije, C. A., Gootenberg, J. S., Abudayyeh, O. O., Metsky, H. C., Durbin, A. F., … Sabeti, P. C. (2018). Field-deployable viral diagnostics using CRISPR-Cas13. In Science (Vol. 360). https://doi.org/10.1126/science.aas8836 ()
- : Nair, G., Rebolledo, M., Clinton White, A., Crannell, Z., Rebecca Richards-Kortum, R., Elizabeth Pinilla, A., … Castellanos-Gonzalez, A. (2015). Detection of entamoeba histolytica by recombinase polymerase amplification. American Journal of Tropical Medicine and Hygiene, 93(3), 591–595. https://doi.org/10.4269/ajtmh.15-0276 ()
- : Nawattanapaiboon, K., Prombun, P., Santanirand, P., Vongsakulyanon, A., Srikhirin, T., Sutapun, B., & Kiatpathomchai, W. (2016). Hemoculture and Direct Sputum Detection of mecA-Mediated Methicillin-Resistant Staphylococcus aureus by Loop-Mediated Isothermal Amplification in Combination With a Lateral-Flow Dipstick. Journal of Clinical Laboratory Analysis, 30(5), 760–767. https://doi.org/10.1002/jcla.21935 ()
- : Pecchia, S., & Da Lio, D. (2018). Development of a rapid PCR-Nucleic Acid Lateral Flow Immunoassay (PCR-NALFIA) based on rDNA IGS sequence analysis for the detection of Macrophomina phaseolina in soil. Journal of Microbiological Methods, 151, 118–128. https://doi.org/10.1016/j.mimet.2018.06.010 ()
- : Peng, H., Long, H., Huang, W., Liu, J., Cui, J., Kong, L., Hu, X., Gu, J., & Peng, D. (2017). Rapid, simple and direct detection of Meloidogyne hapla from infected root galls using loop-mediated isothermal amplification combined with FTA technology. Scientific reports, 7, 44853. https://doi.org/10.1038/srep44853 ()
- : Peng, Y., Zheng, X., Kan, B., Li, W., Zhang, W., Jiang, T., … Qin, A. (2019). Rapid detection of Burkholderia pseudomallei with a lateral flow recombinase polymerase amplification assay. PLoS ONE, 14(7). https://doi.org/10.1371/journal.pone.0213416 ()
- : Qi, Y., Shao, Y., Rao, J., Shen, W., Yin, Q., Li, X., … Li, Y. (2018). Development of a rapid and visual detection method for Rickettsia rickettsii combining recombinase polymerase assay with lateral flow test. PLoS ONE, 13(11). https://doi.org/10.1371/journal.pone.0207811 ()
- : Qian, J., Boswell, S. A., Chidley, C., Lu, Z. xiang, Pettit, M. E., Gaudio, B. L., … Springer, M. (2020). An enhanced isothermal amplification assay for viral detection. Nature Communications, 11(1). https://doi.org/10.1038/s41467-020-19258-y ()
- : Rames, E. K., & Macdonald, J. (2019). Rapid assessment of viral water quality using a novel recombinase polymerase amplification test for human adenovirus. Applied Microbiology and Biotechnology, 103(19), 8115–8125. https://doi.org/10.1007/s00253-019-10077-w ()
- : Sagcan, H., & Turgut Kara, N. (2019). Detection of Potato ring rot Pathogen Clavibacter michiganensis subsp. sepedonicus by Loop-mediated isothermal amplification (LAMP) assay. Scientific Reports, 9(1). https://doi.org/10.1038/s41598-019-56680-9 ()
- : Saldarriaga, O. A., Castellanos-Gonzalez, A., Porrozzi, R., Baldeviano, G. C., Lescano, A. G., de Los Santos, M. B., … Travi, B. L. (2016). An Innovative Field-Applicable Molecular Test to Diagnose Cutaneous Leishmania Viannia spp. Infections. PLoS Neglected Tropical Diseases, 10(4). https://doi.org/10.1371/journal.pntd.0004638 ()
- : Saxena, A., Pal, V., Tripathi, N. K., & Goel, A. K. (2019). Development of a rapid and sensitive recombinase polymerase amplification-lateral flow assay for detection of Burkholderia mallei. Transboundary and Emerging Diseases, 66(2), 1016–1022. https://doi.org/10.1111/tbed.13126 ()
- : Soliman, H., & El-Matbouli, M. (2018). Rapid detection and differentiation of carp oedema virus and cyprinid herpes virus-3 in koi and common carp. Journal of Fish Diseases, 41(5), 761–772. https://doi.org/10.1111/jfd.12774 ()
- : Srisrattakarn, A., Tippayawat, P., Chanawong, A., Tavichakorntrakool, R., Daduang, J., Wonglakorn, L., … Lulitanond, A. (2020). Direct detection of methicillin-resistant in Staphylococcus spp. in positive blood culture by isothermal recombinase polymerase amplification combined with lateral flow dipstick assay. World Journal of Microbiology and Biotechnology, 36(11). https://doi.org/10.1007/s11274-020-02938-8 ()
- : Szántó-Egész, R., Jánosi, A., Mohr, A., Szalai, G., Szabó, E. K., Micsinai, A., … Zsolnai, A. (2016). Breed-Specific Detection of Mangalica Meat in Food Products. Food Analytical Methods, 9(4), 889–894. https://doi.org/10.1007/s12161-015-0261-0 ()
- : Tsou, J. H., Leng, Q., & Jiang, F. (2019). A CRISPR Test for Detection of Circulating Nuclei Acids. Translational Oncology, 12(12), 1566–1573. https://doi.org/10.1016/j.tranon.2019.08.011 ()
- : Yongkiettrakul, S., Jaroenram, W., Arunrut, N., Chareanchim, W., Pannengpetch, S., Suebsing, R., … Kongkasuriyachai, D. (2014). Application of loop-mediated isothermal amplification assay combined with lateral flow dipstick for detection of Plasmodium falciparum and Plasmodium vivax. Parasitology International, 63(6), 777–784. https://doi.org/10.1016/j.parint.2014.06.004 ()
- : Yongkiettrakul, S., Kolié, F. R., Kongkasuriyachai, D., Sattabongkot, J., Nguitragool, W., Nawattanapaibool, N., … Buates, S. (2020). Validation of PfSNP-LAMP-Lateral Flow Dipstick for Detection of Single Nucleotide Polymorphism Associated with Pyrimethamine Resistance in Plasmodium falciparum. Diagnostics, 10(11), 948. https://doi.org/10.3390/diagnostics10110948 ()
- : Yu, J., Shen, D., Dai, T., Lu, X., Xu, H., & Dou, D. (2019). Rapid and equipment-free detection of Phytophthora capsici using lateral flow strip-based recombinase polymerase amplification assay. Letters in Applied Microbiology, 69(1), 64–70. https://doi.org/10.1111/lam.13166 ()
- : Zaky, W. I., Tomaino, F. R., Pilotte, N., Laney, S. J., & Williams, S. A. (2018). Backpack PCR: A point-of-collection diagnostic platform for the rapid detection of Brugia parasites in mosquitoes. PLoS Neglected Tropical Diseases, 12(11). https://doi.org/10.1371/journal.pntd.0006962 ()
- : Zhai, Y., Ma, P., Fu, X., Zhang, L., Cui, P., Li, H., … Yang, X. (2020). A recombinase polymerase amplification combined with lateral flow dipstick for rapid and specific detection of African swine fever virus. Journal of Virological Methods, 285. https://doi.org/10.1016/j.jviromet.2020.113885 ()
- : Zhao, C., Sun, F., Li, X., Lan, Y., Du, L., Zhou, T., & Zhou, Y. (2019). Reverse transcription-recombinase polymerase amplification combined with lateral flow strip for detection of rice black-streaked dwarf virus in plants. Journal of Virological Methods, 263, 96–100. https://doi.org/10.1016/j.jviromet.2018.11.001 ()
- : Zhao, G., Hou, P., Huan, Y., He, C., Wang, H., & He, H. (2018). Development of a recombinase polymerase amplification combined with a lateral flow dipstick assay for rapid detection of the Mycoplasma bovis. BMC Veterinary Research, 14(1). https://doi.org/10.1186/s12917-018-1703-x ()
- : Baerwald, M. R., Goodbla, A. M., Nagarajan, R. P., Gootenberg, J. S., Abudayyeh, O. O., Zhang, F., & Schreier, A. D. (2020). Rapid and accurate species identification for ecological studies and monitoring using CRISPR-based SHERLOCK. Molecular ecology resources, 20(4), 961–970. https://doi.org/10.1111/1755-0998.13186 ()
- : Wang, X., Xiong, E., Tian, T., Cheng, M., Lin, W., Wang, H., Zhang, G., Sun, J., & Zhou, X. (2020). Clustered Regularly Interspaced Short Palindromic Repeats/Cas9-Mediated Lateral Flow Nucleic Acid Assay. ACS nano, 14(2), 2497–2508. https://doi.org/10.1021/acsnano.0c00022 ()
- : Fischer, C., Wessels, H., Paschke-Kratzin, A., & Fischer, M. (2017). Aptamers: Universal capture units for lateral flow applications. Analytical biochemistry, 522, 53–60. https://doi.org/10.1016/j.ab.2017.01.012 ()
- : Kim, T. H., Park, J., Kim, C. J., & Cho, Y. K. (2014). Fully integrated lab-on-a-disc for nucleic acid analysis of food-borne pathogens. Analytical chemistry, 86(8), 3841–3848. https://doi.org/10.1021/ac403971h ()
- : Li, J., Macdonald, J., & von Stetten, F. (2018). Review: a comprehensive summary of a decade development of the recombinase polymerase amplification. The Analyst, 144(1), 31–67. https://doi.org/10.1039/c8an01621f ()
- : Nguyen, P. Q., Soenksen, L. R., Donghia, N. M., Angenent-Mari, N. M., de Puig, H., Huang, A., Lee, R., Slomovic, S., Galbersanini, T., Lansberry, G., Sallum, H. M., Zhao, E. M., Niemi, J. B., & Collins, J. J. (2021). Wearable materials with embedded synthetic biology sensors for biomolecule detection. Nature biotechnology, 39(11), 1366–1374. https://doi.org/10.1038/s41587-021-00950-3 ()




