Entamoeba histolytica - IgG ELISA
This ELISA kit is intended for the qualitative detection of IgG antibodies against Entamoeba histolytica in human serum.
IVDR certified
This product is manufactured by Bordier Affinity Products in Switzerland and distributed in Germany exclusively by Milenia Biotec.
This ELISA kit is intended for the qualitative detection of IgG antibodies against Entamoeba histolytica in human serum.
IVDR certified
This product is manufactured by Bordier Affinity Products in Switzerland and distributed in Germany exclusively by Milenia Biotec.
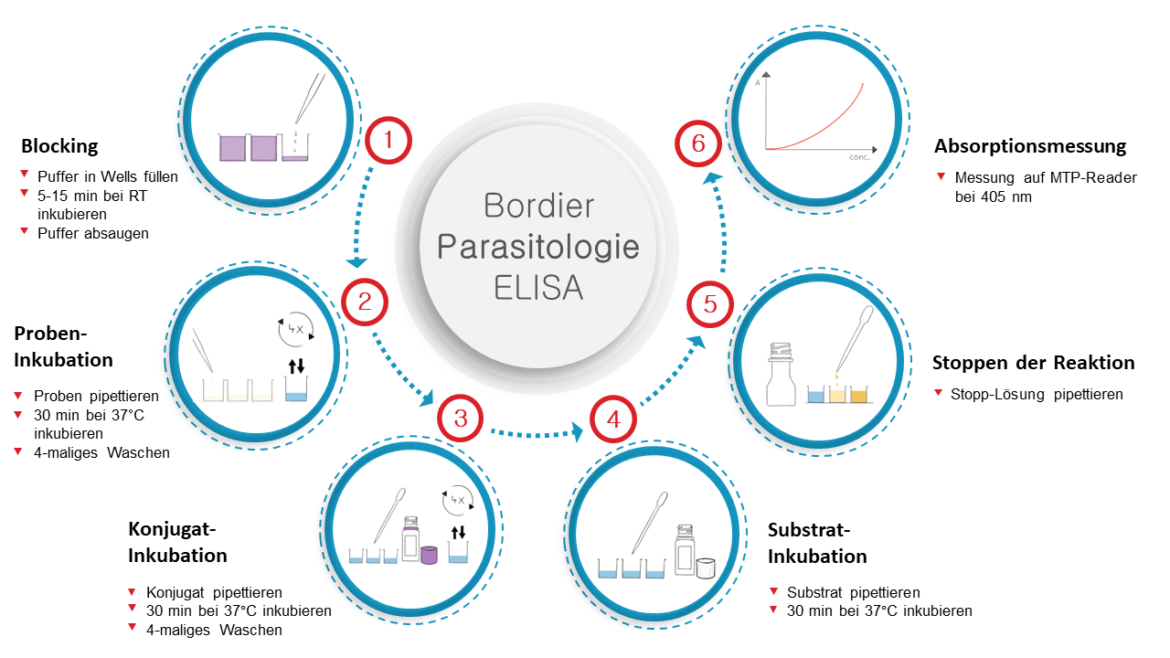
Specific antibodies in the sample bind to Entamoeba histolytica antigens sensitized on microtiter plates. The presence of parasite specific antibodies is detected with a Protein A – alkaline phosphatase conjugate.
2-8 °C
ELISA wells, dilution buffer, washing solution, control sera, conjugate, substrate solution, enzyme buffer, stopping solution
-
Diagnostic methods for differentiation of E. histolytica and E. dispar in carriers : performance and clinical implications in a non-endemic setting. Int. J. Med. Microbiol. 296, 397-403. (2006)
Visser, L.G., Verweij, J.J., Van Esbroeck, M., Edeling, W.M., Clerinx, J. and Polderman, A.M.
-
Standardization and evaluation of ELISA for the serodiagnostic of amoebic liver abscess. Mem. Inst. Oswaldo Cruz, Rio de Janeiro. 89, 53-58. (1994)
Nicholls, R.S., I Restrepo, M., Duque, S., Consuelo Lopez, M. and Corredor A.