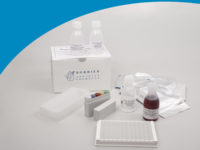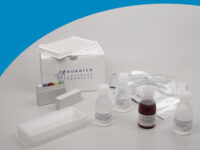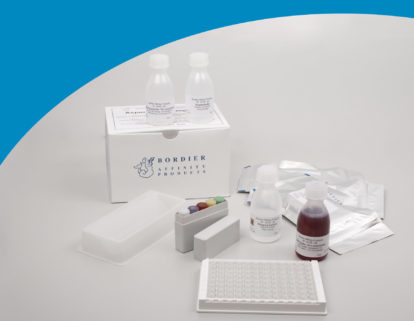
Ascaris - IgG ELISA
This ELISA kit is intended for the qualitative detection of IgG antibodies against parasites of Ascaris genus in human serum.
IVDR certified
This product is manufactured by Bordier Affinity Products in Switzerland and distributed in Germany exclusively by Milenia Biotec.
This ELISA kit is intended for the qualitative detection of IgG antibodies against parasites of Ascaris genus in human serum.
IVDR certified
This product is manufactured by Bordier Affinity Products in Switzerland and distributed in Germany exclusively by Milenia Biotec.
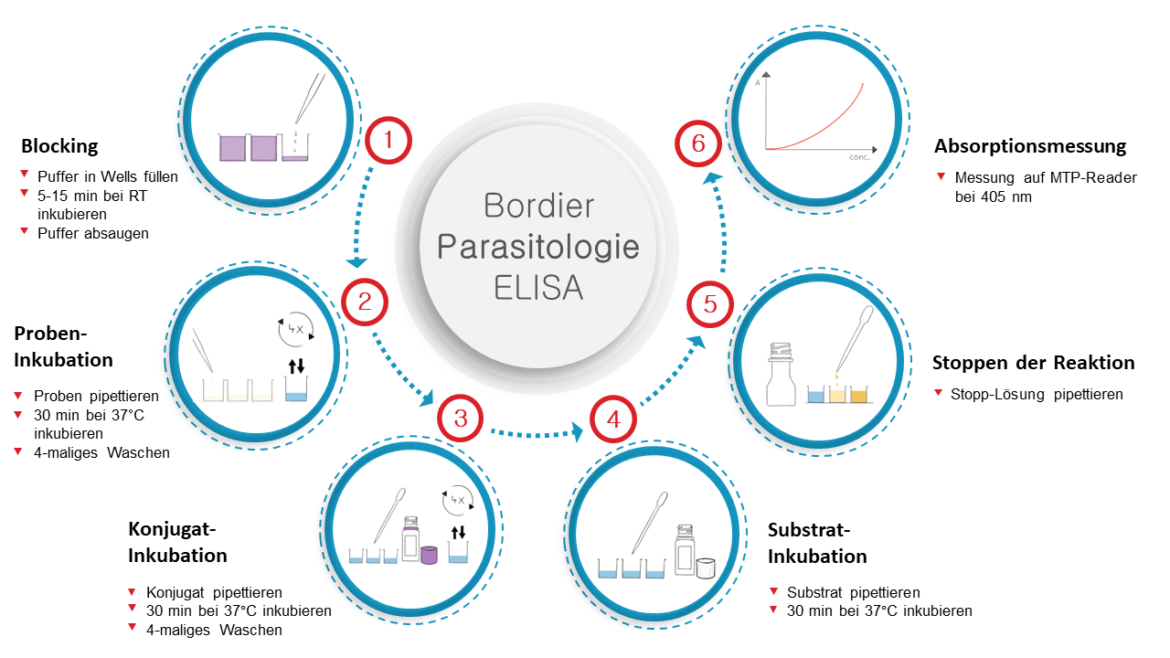
Specific antibodies in the sample bind to Ascaris solubles coelomics antigens. The presence of parasite specific antibodies is detected with a Protein A alkaline phosphatase conjugate.
2-8 °C
ELISA wells, dilution buffer, washing solution, control sera, conjugate, substrate solution, stopping solution
-
Evaluation of copromicroscopy and serology to measure the exposure to Ascaris infections across age groups and to assess the impact of 3 years of biannual mass drug administration in Jimma Town, Ethiopia (2020)
Dana, D., Vlaminck, J., Tadege, B., Mekonnen, Z., Geldhof, P. et al.
-
Incidence of Ascaris suum-specific antibodies in Austian patients with suspected larva migrans visceralis (VLM) syndrome (2016)
Schneider, R. and Auer, H.